Automated analysis of filopodial length and spatially resolved protein concentration via adaptive shape tracking
- PMID: 27535428
- PMCID: PMC5221593
- DOI: 10.1091/mbc.E16-06-0406
Automated analysis of filopodial length and spatially resolved protein concentration via adaptive shape tracking
Abstract
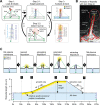
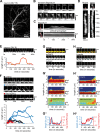
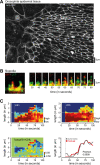
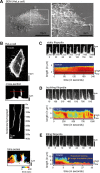
Filopodia are dynamic, actin-rich structures that transiently form on a variety of cell types. To understand the underlying control mechanisms requires precise monitoring of localization and concentration of individual regulatory and structural proteins as filopodia elongate and subsequently retract. Although several methods exist that analyze changes in filopodial shape, a software solution to reliably correlate growth dynamics with spatially resolved protein concentration along the filopodium independent of bending, lateral shift, or tilting is missing. Here we introduce a novel approach based on the convex-hull algorithm for parallel analysis of growth dynamics and relative spatiotemporal protein concentration along flexible filopodial protrusions. Detailed in silico tests using various geometries confirm that our technique accurately tracks growth dynamics and relative protein concentration along the filopodial length for a broad range of signal distributions. To validate our technique in living cells, we measure filopodial dynamics and quantify spatiotemporal localization of filopodia-associated proteins during the filopodial extension-retraction cycle in a variety of cell types in vitro and in vivo. Together these results show that the technique is suitable for simultaneous analysis of growth dynamics and spatiotemporal protein enrichment along filopodia. To allow readily application by other laboratories, we share source code and instructions for software handling.
© 2016 Saha et al. This article is distributed by The American Society for Cell Biology under license from the author(s). Two months after publication it is available to the public under an Attribution–Noncommercial–Share Alike 3.0 Unported Creative Commons License (http://creativecommons.org/licenses/by-nc-sa/3.0).
Figures


References
-
- Abramoff MD, Magalhaes PJ, Ram SJ. Image processing with ImageJ. Biophotonics Int. 2004;11:36–42.
-
- Argiro V, Bunge MB, Johnson MI. A quantitative study of growth cone filopodial extension. J Neurosci Res. 1985;13:149–162. - PubMed
-
- Best A, Ahmed S, Kozma R, Lim L. The Ras-related GTPase Rac1 binds tubulin. J Biol Chem. 1996;271:3756–3762. - PubMed
-
- Blum H. A transformation for extracting new descriptors of shape. In: Wathen-Dunn W, editor. Models for the Perception of Speech and Visual Form: Proceedings of a Symposium; Cambridge, MA. MIT Press; 1967. pp. 362–380.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

