PROGRESS - prospective observational study on hospitalized community acquired pneumonia
- PMID: 27535544
- PMCID: PMC4987996
- DOI: 10.1186/s12890-016-0255-8
PROGRESS - prospective observational study on hospitalized community acquired pneumonia
Abstract
Background: Community acquired pneumonia (CAP) is a high incidence disease resulting in about 260,000 hospital admissions per year in Germany, more than myocardial infarction or stroke. Worldwide, CAP is the most frequent infectious disease with high lethality ranging from 1.2 % in those 20-29 years old to over 10 % in patients older than 70 years, even in industrial nations. CAP poses numerous medical challenges, which the PROGRESS (Pneumonia Research Network on Genetic Resistance and Susceptibility for the Evolution of Severe Sepsis) network aims to tackle: Operationalization of disease severity throughout the course of disease, outcome prediction for hospitalized patients and prediction of transitions from uncomplicated CAP to severe CAP, and finally, to CAP with sepsis and organ failure as a life-threatening condition. It is a major aim of PROGRESS to understand and predict patient heterogeneity regarding outcome in the hospital and to develop novel treatment concepts.
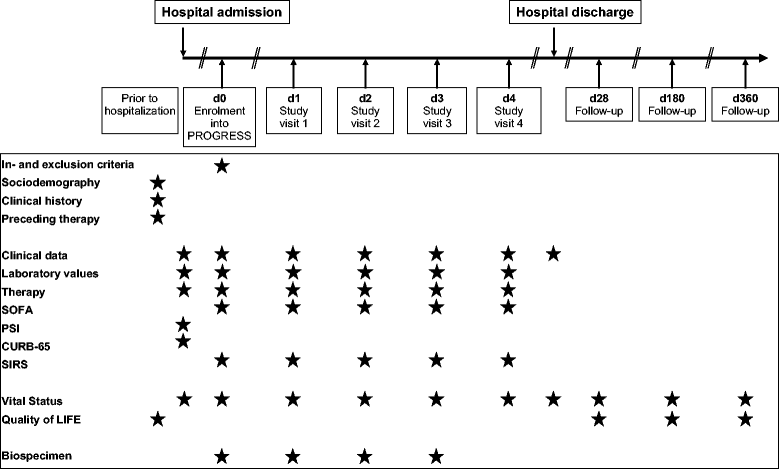
Methods: PROGRESS was designed as a clinical, observational, multi-center study of patients with CAP requiring hospitalization. More than 1600 patients selected for low burden of co-morbidities have been enrolled, aiming at a total of 3000. Course of disease, along with therapy, was closely monitored by daily assessments and long-term follow-up. Daily blood samples allow in depth molecular-genetic characterization of patients. We established a well-organized workflow for sample logistics and a comprehensive data management system to collect and manage data from more than 50 study centers in Germany and Austria. Samples are stored in a central biobank and clinical data are stored in a central data base which also integrates all data from molecular assessments.
Discussion: With the PROGRESS study, we established a comprehensive data base of high quality clinical and molecular data allowing investigation of pressing research questions regarding CAP. In-depth molecular characterization will contribute to the discovery of disease mechanisms and establishment of diagnostic and predictive biomarkers. A strength of PROGRESS is the focus on younger patients with low burden of co-morbidities, allowing a more direct look at host biology with less confounding. As a resulting limitation, insights from PROGRESS will require validation in representative patient cohorts to assess clinical utility.
Trial registration: The PROGRESS study was retrospectively registered on May 24(th), 2016 with ClinicalTrials.gov: NCT02782013.
Keywords: Biobank; Biomarkers; Data base; Disease progression; Innate immunity; Pneumonia; Prospective observational study; Sepsis.
Figures
References
-
- Angus DC, Marrie TJ, Obrosky DS, Clermont G, Dremsizov TT, Coley C, et al. Severe community-acquired pneumonia: use of intensive care services and evaluation of American and British Thoracic Society Diagnostic criteria. Am J Respir Crit Care Med. 2002;166(5):717–23. doi: 10.1164/rccm.2102084. - DOI - PubMed
-
- AQUA-Institut . Bundesauswertung zum Erfassungsjahr 2014. PNEU – Ambulant erworbene Pneumonie. Qualitätsindikatoren. 2015.
-
- Brunkhorst FM; Engel C, Bone HG, Brunkhorst R, Gerlach H, Grond S et al. Epidemiology of severe sepsis and septic shock in Germany - Results from the German Prevalence Study. Infection. 2005;33(Suppl. 1):49.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

