Plerixafor (a CXCR4 antagonist) following myeloablative allogeneic hematopoietic stem cell transplantation enhances hematopoietic recovery
- PMID: 27535663
- PMCID: PMC4989381
- DOI: 10.1186/s13045-016-0301-2
Plerixafor (a CXCR4 antagonist) following myeloablative allogeneic hematopoietic stem cell transplantation enhances hematopoietic recovery
Abstract
Background: The binding of CXCR4 with its ligand (stromal-derived factor-1) maintains hematopoietic stem/progenitor cells (HSPCs) in a quiescent state. We hypothesized that blocking CXCR4/SDF-1 interaction after hematopoietic stem cell transplantation (HSCT) promotes hematopoiesis by inducing HSC proliferation.
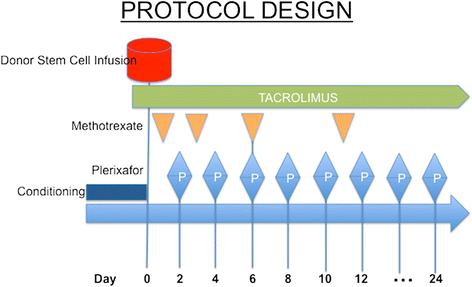
Methods: We conducted a phase I/II trial of plerixafor on hematopoietic cell recovery following myeloablative allogeneic HSCT. Patients with hematologic malignancies receiving myeloablative conditioning were enrolled. Plerixafor 240 μg/kg was administered subcutaneously every other day beginning day +2 until day +21 or until neutrophil recovery. The primary efficacy endpoints of the study were time to absolute neutrophil count >500/μl and platelet count >20,000/μl. The cumulative incidence of neutrophil and platelet engraftment of the study cohort was compared to that of a cohort of 95 allogeneic peripheral blood stem cell transplant recipients treated during the same period of time and who received similar conditioning and graft-versus-host disease prophylaxis.
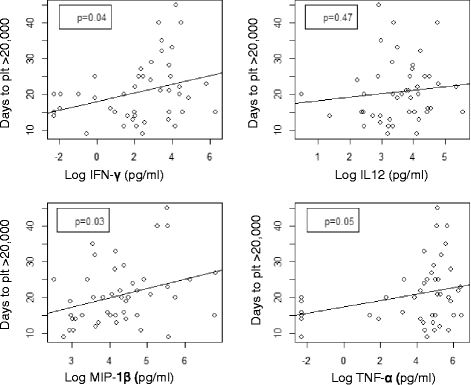
Results: Thirty patients received plerixafor following peripheral blood stem cell (n = 28) (PBSC) or bone marrow (n = 2) transplantation. Adverse events attributable to plerixafor were mild and indistinguishable from effects of conditioning. The kinetics of neutrophil and platelet engraftment, as demonstrated by cumulative incidence, from the 28 study subjects receiving PBSC showed faster neutrophil (p = 0.04) and platelet recovery >20 K (p = 0.04) compared to the controls.
Conclusions: Our study demonstrated that plerixafor can be given safely following myeloablative HSCT. It provides proof of principle that blocking CXCR4 after HSCT enhances hematopoietic recovery. Larger, confirmatory studies in other settings are warranted.
Trial registration: ClinicalTrials.gov NCT01280955.
Keywords: Antagonist; CXCR4; Hematopoietic stem cell transplantation; Hematopoietic stem cells; Neutrophil engraftment; Outcomes; Platelet engraftment; Stromal-derived factor-1.
Figures


Similar articles
-
Influence of a dual-injection regimen, plerixafor and CXCR4 on in utero hematopoietic stem cell transplantation and engraftment with use of the sheep model.Cytotherapy. 2014 Sep;16(9):1280-93. doi: 10.1016/j.jcyt.2014.05.025. Cytotherapy. 2014. PMID: 25108653 Free PMC article.
-
Hematopoietic stem cell transplantation using single UM171-expanded cord blood: a single-arm, phase 1-2 safety and feasibility study.Lancet Haematol. 2020 Feb;7(2):e134-e145. doi: 10.1016/S2352-3026(19)30202-9. Epub 2019 Nov 6. Lancet Haematol. 2020. PMID: 31704264 Clinical Trial.
-
Post-transplantation Cyclophosphamide and Sirolimus after Haploidentical Hematopoietic Stem Cell Transplantation Using a Treosulfan-based Myeloablative Conditioning and Peripheral Blood Stem Cells.Biol Blood Marrow Transplant. 2015 Aug;21(8):1506-14. doi: 10.1016/j.bbmt.2015.04.025. Epub 2015 May 19. Biol Blood Marrow Transplant. 2015. PMID: 26001696
-
Alloreactivity as therapeutic principle in the treatment of hematologic malignancies. Studies of clinical and immunologic aspects of allogeneic hematopoietic cell transplantation with nonmyeloablative conditioning.Dan Med Bull. 2007 May;54(2):112-39. Dan Med Bull. 2007. PMID: 17521527 Review.
-
Plerixafor: A chemokine receptor-4 antagonist for mobilization of hematopoietic stem cells for transplantation after high-dose chemotherapy for non-Hodgkin's lymphoma or multiple myeloma.Clin Ther. 2010 May;32(5):821-43. doi: 10.1016/j.clinthera.2010.05.007. Clin Ther. 2010. PMID: 20685493 Review.
Cited by
-
Role of CXCR4 in the progression and therapy of acute leukaemia.Cell Prolif. 2021 Jul;54(7):e13076. doi: 10.1111/cpr.13076. Epub 2021 May 29. Cell Prolif. 2021. PMID: 34050566 Free PMC article. Review.
-
PPAR agonists attenuate lenalidomide's anti-myeloma activity in vitro and in vivo.Cancer Lett. 2022 Oct 1;545:215832. doi: 10.1016/j.canlet.2022.215832. Epub 2022 Jul 22. Cancer Lett. 2022. PMID: 35872263 Free PMC article.
-
Identification of CXCR4 and CXCL10 as Potential Predictive Biomarkers in Triple Negative Breast Cancer (TNBC).Med Sci Monit. 2020 Jan 11;26:e918281. doi: 10.12659/MSM.918281. Med Sci Monit. 2020. PMID: 31924747 Free PMC article.
-
Hematopoietic Stem Cell Niche During Homeostasis, Malignancy, and Bone Marrow Transplantation.Front Cell Dev Biol. 2021 Jan 22;9:621214. doi: 10.3389/fcell.2021.621214. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33553181 Free PMC article. Review.
-
Targeting CXCR4 in AML and ALL.Front Oncol. 2020 Sep 4;10:1672. doi: 10.3389/fonc.2020.01672. eCollection 2020. Front Oncol. 2020. PMID: 33014834 Free PMC article. Review.
References
-
- Baron F, Labopin M, Ruggeri A, Mohty M, Sanz G, Milpied N, Bacigalupo A, Rambaldi A, Bonifazi F, Bosi A, et al. Unrelated cord blood transplantation for adult patients with acute myeloid leukemia: higher incidence of acute graft-versus-host disease and lower survival in male patients transplanted with female unrelated cord blood—a report from Eurocord, the Acute Leukemia Working Party, and the Cord Blood Committee of the Cellular Therapy and Immunobiology Working Party of the European Group for Blood and Marrow Transplantation. J Hematol Oncol. 2015;8:107. doi: 10.1186/s13045-015-0207-4. - DOI - PMC - PubMed
-
- Lai YR, Chen YH, Hu DM, Jiang M, Liu QF, Liu L, Hou J, Schwarzenberger P, Li QC, Zhang ZM, et al. Multicenter phase II study of a combination of cyclosporine a, methotrexate and mycophenolate mofetil for GVHD prophylaxis: results of the Chinese Bone Marrow Transplant Cooperative Group (CBMTCG) J Hematol Oncol. 2014;7:59. doi: 10.1186/s13045-014-0059-3. - DOI - PMC - PubMed
-
- Young JH, Logan BR, Wu J, Wingard JR, Weisdorf DJ, Mudrick C, Knust K, Horowitz MM, Confer DL, Dubberke ER, et al. Infections after transplantation of bone marrow or peripheral blood stem cells from unrelated donors. Biol Blood Marrow Transplant. 2016;22:359–370. doi: 10.1016/j.bbmt.2015.09.013. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

