Mechanosensing by the α6-integrin confers an invasive fibroblast phenotype and mediates lung fibrosis
- PMID: 27535718
- PMCID: PMC4992155
- DOI: 10.1038/ncomms12564
Mechanosensing by the α6-integrin confers an invasive fibroblast phenotype and mediates lung fibrosis
Abstract
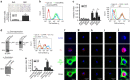
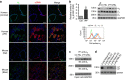
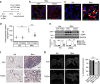
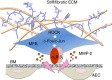
Matrix stiffening is a prominent feature of pulmonary fibrosis. In this study, we demonstrate that matrix stiffness regulates the ability of fibrotic lung myofibroblasts to invade the basement membrane (BM). We identify α6-integrin as a mechanosensing integrin subunit that mediates matrix stiffness-regulated myofibroblast invasion. Increasing α6-expression, specifically the B isoform (α6B), couples β1-integrin to mediate MMP-2-dependent pericellular proteolysis of BM collagen IV, leading to myofibroblast invasion. Human idiopathic pulmonary fibrosis lung myofibroblasts express high levels of α6-integrin in vitro and in vivo. Genetic ablation of α6 in collagen-expressing mesenchymal cells or pharmacological blockade of matrix stiffness-regulated α6-expression protects mice against bleomycin injury-induced experimental lung fibrosis. These findings suggest that α6-integrin is a matrix stiffness-regulated mechanosensitive molecule which confers an invasive fibroblast phenotype and mediates experimental lung fibrosis. Targeting this mechanosensing α6(β1)-integrin offers a novel anti-fibrotic strategy against lung fibrosis.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

