Endothelin receptor antagonists for persistent pulmonary hypertension in term and late preterm infants
- PMID: 27535894
- PMCID: PMC8588275
- DOI: 10.1002/14651858.CD010531.pub2
Endothelin receptor antagonists for persistent pulmonary hypertension in term and late preterm infants
Abstract
Background: Endothelin, a powerful vasoconstrictor, is one of the mediators in the causation of persistent pulmonary hypertension of the newborn (PPHN). Theoretically, endothelin receptor antagonists (ETRA) have the potential to improve the outcomes of infants with PPHN.
Objectives: To assess the efficacy and safety of ETRA in the treatment of PPHN in full-term, post-term and late preterm infants.To assess the efficacy and safety of selective ETRAs (which block only the ETA receptors) and non-selective ETRAs (which block both ETA and ETB receptors) separately.
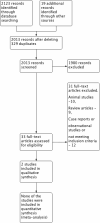
Search methods: CENTRAL (Cochrane Central Register of Controlled Trials), MEDLINE, EMBASE and CINAHL databases were searched until December 2015.
Selection criteria: Randomised, cluster-randomised or quasi-randomised controlled trials were eligible.
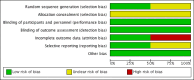
Data collection and analysis: Two review authors independently searched the literature, selected the studies, assessed the risk of bias and extracted the data. A fixed-effect model was used for meta-analysis. We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to assess the quality of evidence.
Main results: Two randomised controlled trials of ETRA met the inclusion criteria. Both studies utilized oral Bosentan. The first study was done in a setting where inhaled nitric oxide (iNO) therapy was not available. Forty-seven infants (≥ 34 weeks' gestation) were randomised to receive either Bosentan or placebo. The second study was a multicentre study where iNO therapy was the standard of care for PPHN. Twenty-one infants were randomised to receive either 'iNO plus Bosentan' or 'iNO plus placebo'.In the first study, there was no significant difference in the incidence of death before hospital discharge between the Bosentan and placebo groups (1/23 vs 3/14; RR 0.20, 95% CI 0.02 to 1.77; RD -0.17, 95% CI -0.40 to 0.06). A higher proportion of infants in the Bosentan group showed improvement in oxygenation index (OI) at the end of therapy (21/24 vs 3/15; RR 4.38, 95% CI 1.57 to 12.17; RD 0.68, 95% CI 0.43 to 0.92; number needed to treat for a beneficial outcome (NNTB) 1.5). The duration of mechanical ventilation was lower in the Bosentan group (4.3 ± 0.9 vs 11.5 ± 0.6 days; MD -7.20, 95% CI -7.64 to -6.76). There was no significant difference in adverse neurological outcomes at six months (0/23 vs 4/14; RR 0.07, 95% CI 0.00 to 1.20; RD -0.29, 95% CI -0.52 to -0.05). The study suffered from a high risk of attrition bias since 8/23 infants in the placebo group were excluded from various analyses. Since the protocol for the study could not be accessed, the study suffered from unclear risk of reporting bias.In the second study, there was no significant difference in the incidence of treatment failure needing extracorporeal membrane oxygenation (ECMO) between the 'iNO plus Bosentan' vs 'iNO plus placebo' groups (1/13 vs 0/8; RR 1.93, 95% CI 0.09 to 42.35; RD 0.08, 95% CI -0.14 to 0.30). There was no significant difference in the median time to wean from iNO ('iNO plus Bosentan': 3.7 days (95% CI 1.17 to 6.95); 'iNO plus placebo': 2.9 days (95% CI 1.26 to 4.23); P = 0.34). There were no significant differences in the OI 0, 3, 5, 12, 24, 48 and 72 hours of treatment between the groups. There were no significant differences in the time to complete weaning from mechanical ventilation (median 10.8 days (CI 3.21 to 12.21) versus 8.6 days (CI 3.71 to 9.66); P = 0.24). The study had unequal distribution to the Bosentan group (N = 13) and the placebo group (N = 8). The methods used for generating random sequence numbers and allocation concealment were unclear, resulting in unclear risk of selection bias.Both studies reported that Bosentan was well tolerated and no major adverse effects were noted. Data from the two studies was not pooled given the heterogenous nature of the clinical settings and the modalities used for the treatment of PPHN.Overall, the quality of evidence was considered low, given the small sample size of the included studies, the numerical imbalance between the groups due to randomisation and attrition, and unclear risk of bias on some of the important domains.
Authors' conclusions: There is inadequate evidence to support the use of ETRAs either as stand-alone therapy or as adjuvant to inhaled nitric oxide in PPHN. Adequately powered RCTs are needed.
Conflict of interest statement
Kiran More: none known
Gayatri Athalye‐Jape: none known
Shripada Rao: none known
Sanjay Patole: none known
Figures

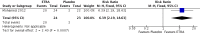

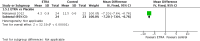











Update of
References
References to studies included in this review
Mohamed 2012 {published data only}
-
- Mohamed WA, Ismail M. A randomized, double‐blind, placebo‐controlled, prospective study of bosentan for the treatment of persistent pulmonary hypertension of the newborn. Journal of Perinatology 2012;32(8):608‐13. - PubMed
Steinhorn 2014 {published data only}
-
- NCT01389856: Persistent Pulmonary Hypertension of the Newborn (FUTURE 4). https://clinicaltrials.gov/ct2/show/results/NCT01389856?sect=X01256#all.
-
- Steinhorn R, Kusic‐Pajic A, Cornelisse P, Fineman J, Gehin M, Nowbakht P, et al. Bosentan as adjunctive therapy for persistent pulmonary hypertension of the newborn: Results of the FUTURE‐4 study. Circulation: American Heart Association's 2014 Scientific Sessions and Resuscitation Science Symposium Chicago, IL United States. 2014.
Additional references
Abman 2007
-
- Abman SH. Recent advances in the pathogenesis and treatment of persistent pulmonary hypertension of the newborn. Neonatology 2007;91(4):283‐90. - PubMed
Abman 2009
-
- Abman SH. Role of endothelin receptor antagonists in the treatment of pulmonary arterial hypertension. Annual Review of Medicine 2009;60:13‐23. - PubMed
Allen 1993
-
- Allen SW, Chatfield BA, Koppenhafer SA, Schaffer MS, Wolfe RR, Abman SH. Circulating immunoreactive endothelin‐1 in children with pulmonary hypertension. Association with acute hypoxic pulmonary vasoreactivity. American Review of Respiratory Disease 1993;148(2):519‐22. - PubMed
Ambalavanan 2005
Barton 2008
-
- Barton M, Yanagisawa M. Endothelin: 20 years from discovery to therapy. Canadian Journal of Physiology and Pharmacology 2008;86(8):485‐98. - PubMed
Beghetti 2009
-
- Beghetti M. Bosentan in pediatric patients with pulmonary arterial hypertension. Current Vascular Pharmacology 2009;7(2):225‐33. - PubMed
Dakshinamurti 2005
-
- Dakshinamurti S. Pathophysiologic mechanisms of persistent pulmonary hypertension of the newborn. Pediatric Pulmonology 2005;39(6):492‐503. - PubMed
Davenport 2006
-
- Davenport AP, Maguire JJ. Endothelin. Handbook of Experimental Pharmacology 2006;176 Pt 1:295‐329. - PubMed
de Lagausie 2005
-
- Lagausie P, Buys‐Roessingh A, Ferkdadji L, Saada J, Aisenfisz S, Martinez‐Vinson C, et al. Endothelin receptor expression in human lungs of newborns with congenital diaphragmatic hernia. Journal of Pathology 2005;205(1):112‐8. - PubMed
Dhillon 2012
-
- Dhillon R. The management of neonatal pulmonary hypertension. Archives of Diseases in Childhood. Fetal and Neonatal Edition 2012;97(3):F223‐8. - PubMed
Drinker 2012
-
- Drinker LR, Cotten CM, Rattray BN, Hornik CD, Smith PB, Goldberg RN, et al. Bosentan in Neonatal Patients with Pulmonary Hypertension. E‐PAS2012. 2012; Vol. 1533.552.
Egger 1997
Endo 2001
-
- Endo A, Ayusawa M, Minato M, Takada M, Takahashi S, Harada K. Endogenous nitric oxide and endothelin‐1 in persistent pulmonary hypertension of the newborn. European Journal of Pediatrics 2001;160(4):217‐22. - PubMed
Galiè 2004
-
- Galiè N, Manes A, Branzi A. The endothelin system in pulmonary arterial hypertension. Cardiovascular Research 2004;61(2):227‐37. - PubMed
Geiger 2006
-
- Geiger R, Pajk W, Neu N, Maier S, Kleinsasser A, Fratz S, et al. Tezosentan decreases pulmonary artery pressure and improves survival rate in an animal model of meconium aspiration. Pediatric Research 2006;59(1):147‐50. - PubMed
Geiger 2008
-
- Geiger R, Kleinsasser A, Meier S, Neu N, Pajk W, Fischer V, et al. Intravenous tezosentan improves gas exchange and hemodynamics in acute lung injury secondary to meconium aspiration. Intensive Care Medicine 2008;34(2):368‐76. - PubMed
Gersony 1984
-
- Gersony WM. Neonatal pulmonary hypertension: pathophysiology, classification, and etiology. Clinics in Perinatology 1984;11(3):517‐24. - PubMed
Giaid 1993
-
- Giaid A, Yanagisawa M, Langleben D, Michel RP, Levy R, Shennib H, et al. Expression of endothelin‐1 in the lungs of patients with pulmonary hypertension. New England Journal of Medicine 1993;328(24):1732‐9. - PubMed
Goissen 2008
-
- Goissen C, Ghyselen L, Tourneux P, Krim G, Storme L, Bou P, et al. Persistent pulmonary hypertension of the newborn with transposition of the great arteries: successful treatment with bosentan. European Journal of Pediatrics 2008;167(4):437‐40. - PubMed
GRADEpro [Computer program]
-
- McMaster University. GRADEpro [www.gradepro.org]. McMaster University, 2014.
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hirono 2010
-
- Hirono K, Yoshimura N, Taguchi M, Watanabe K, Nakamura T, Ichida F, et al. Bosentan induces clinical and hemodynamic improvement in candidates for right‐sided heart bypass surgery. Journal of Thoracic and Cardiovascular Surgery 2010;140(2):346‐51. - PubMed
Jacobs 2006
-
- Jacobs A, Preston IR, Gomberg‐Maitland M. Endothelin receptor antagonism in pulmonary arterial hypertension‐‐a role for selective ET(A) inhibition?. Current medical research and opinion 2006;22(12):2567‐74. [PUBMED: 17166339] - PubMed
Konduri 2009
Krishnan 2008
-
- Krishnan U, Krishnan S, Gewitz M. Treatment of pulmonary hypertension in children with chronic lung disease with newer oral therapies. Pediatric Cardiology 2008;29(6):1082‐6. - PubMed
Kuo 2001
-
- Kuo CY. Endothelin‐A receptor antagonist prevents neonatal pulmonary hypertension in meconium aspiration in piglets. Journal of the Formosan Medical Association 2001;100(6):420‐3. - PubMed
La 1995
-
- La M, Reid JJ. Endothelin‐1 and the regulation of vascular tone. Clinical and Experimental Pharmacology and Physiology 1995;22(5):315‐23. - PubMed
Lakshminrusimha 1999
-
- Lakshminrusimha S, Steinhorn RH. Pulmonary vascular biology during neonatal transition. Clinics in Perinatology 1999;26(3):601‐19. - PubMed
Levy 2005a
-
- Levy M, Maurey C, Dinh‐Xuan AT, Vouhe P, Israel‐Biet D. Developmental expression of vasoactive and growth factors in human lung. Role in pulmonary vascular resistance adaptation at birth. Pediatric Research 2005;57(5 Pt 2):21R‐5R. - PubMed
Levy 2005b
-
- Levy M, Maurey C, Chailley‐Heu B, Martinovic J, Jaubert F, Israel‐Biet D. Developmental changes in endothelial vasoactive and angiogenic growth factors in the human perinatal lung. Pediatric Research 2005;57(2):248‐53. - PubMed
Liu 2013
Luscher 2000
-
- Luscher TF, Barton M. Endothelins and endothelin receptor antagonists: therapeutic considerations for a novel class of cardiovascular drugs. Circulation 2000;102(19):2434‐40. - PubMed
Moussa 2011
-
- Moussa A, Lavoie J, Barrington KJ. Bosentan for Secondary Pulmonary Arterial Hypertension in Neonates: A Case Series. E‐PAS201129. 2011.
Nakwan 2009
-
- Nakwan N, Choksuchat D, Saksawad R, Thammachote P, Nakwan N. Successful treatment of persistent pulmonary hypertension of the newborn with bosentan. Acta Paediatrica, International Journal of Paediatrics 2009;98(10):1683‐5. - PubMed
Nemoto 2007
-
- Nemoto S, Yoshimura S, Matsumura M, Nishimura K. An initial experience of endotherine‐1 dual‐receptor blockade in the treatment of sustained pulmonary hypertension early after congenital cardiac surgery in infancy. Kyobu Geka. The Japanese Journal of Thoracic Surgery 2007;60(6):453‐6. - PubMed
O'Callaghan 2011
-
- O'Callaghan DS, Savale L, Montani D, Jais X, Sitbon O, Simonneau G, et al. Treatment of pulmonary arterial hypertension with targeted therapies. Nature Reviews. Cardiology 2011;8(9):526‐38. - PubMed
Oishi 2011
-
- Oishi P, Datar SA, Fineman JR. Advances in the management of pediatric pulmonaryhypertension. Respiratory Care 2011;56(9):1314‐39. - PubMed
Pearl 1999
-
- Pearl JM, Wellmann SA, McNamara JL, Lombardi JP, Wagner CJ, Raake JL, et al. Bosentan prevents hypoxia‐reoxygenation‐induced pulmonary hypertension and improves pulmonary function. Annals of Thoracic Surgery 1999;68(5):1714‐21; discussion 1721‐2. - PubMed
Peng 2003
-
- Peng J, Ladino J, Hehre D, Bancalari E, Suguihara E. Effects of a selective endothelin A (ETA) receptor antagonist on GBS‐induced pulmonary hypertension in newborn piglets. Pediatric Academic Societies’ Annual Meeting. 2003; Vol. E‐PAS:2443.
Persson 2009
-
- Persson BP, Rossi P, Weitzberg E, Oldner A. Inhaled tezosentan reduces pulmonary hypertension in endotoxin‐induced lung injury. Shock 2009;32(4):427‐34. - PubMed
Radicioni 2011
-
- Radicioni M, Bruni A, Camerini P. Combination therapy for life‐threatening pulmonary hypertension in a premature infant: First report on bosentan use. European Journal of Pediatrics 2011;170(8):1075‐8. - PubMed
Rao 2010
-
- Rao S, Bartle D, Patole S. Current and future therapeutic options for persistent pulmonary hypertension in the newborn. Expert Review of Cardiovascular Therapy 2010;8(6):845‐62. - PubMed
Roberts 1997
-
- Roberts JD Jr, Fineman JR, Morin FC 3rd, Shaul PW, Rimar S, Schreiber MD, et al. Inhaled nitric oxide and persistent pulmonary hypertension of the newborn. The Inhaled Nitric Oxide Study Group. New England Journal of Medicine 1997;336(9):605‐10. - PubMed
Rosenberg 1993
-
- Rosenberg AA, Kennaugh J, Koppenhafer SL, Loomis M, Chatfield BA, Abman SH. Elevated immunoreactive endothelin‐1 levels in newborn infants with persistent pulmonary hypertension. Journal of Pediatrics 1993;123(1):109‐14. - PubMed
Schünemann 2013
-
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE Working Group. GRADE handbook for grading quality of evidence and strength of recommendations. Available from www.guidelinedevelopment.org/handbook Updated October 2013.
Shah 2011
Shao 2011
-
- Shao D, Park JE, Wort SJ. The role of endothelin‐1 in the pathogenesis of pulmonary arterial hypertension. Pharmacological Research 2011;63(6):504‐11. - PubMed
Stathopoulos 2011
-
- Stathopoulos E, Rolland PH, Héry G, Magnée C, Paut O, Couchot E, et al. Acute effect of a dual ETA‐ETB receptor antagonist on pulmonary arterial vasculature in preterm lamb fetuses with surgically induced diaphragmatic hernia. Pediatric Surgery International 2011;27(3):295‐301. - PubMed
Stayer 2010
-
- Stayer SA, Liu Y. Pulmonary hypertension of the newborn. Best Practice and Research Clinical Anaesthesiology 2010;24(3):375‐86. - PubMed
Steinhorn 2010
Torre‐Amione 2003
-
- Torre‐Amione G, Young JB, Colucci WS, Lewis BS, Pratt C, Cotter G, et al. Hemodynamic and clinical effects of tezosentan, an intravenous dual endothelin receptor antagonist, in patients hospitalized for acute decompensated heart failure. Journal of the American College of Cardiology 2003;42(1):140‐7. - PubMed
Wan 2014
Yanagisawa 1988
-
- Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 1988;332(6163):411‐5. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

