The Brain-Enriched MicroRNA miR-9-3p Regulates Synaptic Plasticity and Memory
- PMID: 27535911
- PMCID: PMC6601897
- DOI: 10.1523/JNEUROSCI.0630-16.2016
The Brain-Enriched MicroRNA miR-9-3p Regulates Synaptic Plasticity and Memory
Abstract
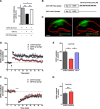
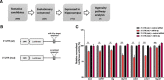
MicroRNAs (miRNAs) are small, noncoding RNAs that posttranscriptionally regulate gene expression in many tissues. Although a number of brain-enriched miRNAs have been identified, only a few specific miRNAs have been revealed as critical regulators of synaptic plasticity, learning, and memory. miR-9-5p/3p are brain-enriched miRNAs known to regulate development and their changes have been implicated in several neurological disorders, yet their role in mature neurons in mice is largely unknown. Here, we report that inhibition of miR-9-3p, but not miR-9-5p, impaired hippocampal long-term potentiation (LTP) without affecting basal synaptic transmission. Moreover, inhibition of miR-9-3p in the hippocampus resulted in learning and memory deficits. Furthermore, miR-9-3p inhibition increased the expression of the LTP-related genes Dmd and SAP97, the expression levels of which are negatively correlated with LTP. These results suggest that miR-9-3p-mediated gene regulation plays important roles in synaptic plasticity and hippocampus-dependent memory.
Significance statement: Despite the abundant expression of the brain-specific microRNA miR-9-5p/3p in both proliferating and postmitotic neurons, most functional studies have focused on their role in neuronal development. Here, we examined the role of miR-9-5p/3p in adult brain and found that miR-9-3p, but not miR-9-5p, has a critical role in hippocampal synaptic plasticity and memory. Moreover, we identified in vivo binding targets of miR-9-3p that are involved in the regulation of long-term potentiation. Our study provides the very first evidence for the critical role of miR-9-3p in synaptic plasticity and memory in the adult mouse.
Keywords: hippocampus; long-term potentiation; memory; microRNA.
Copyright © 2016 the authors 0270-6474/16/368641-12$15.00/0.
Figures




Similar articles
-
NF-κB-regulated microRNA-574-5p underlies synaptic and cognitive impairment in response to atmospheric PM2.5 aspiration.Part Fibre Toxicol. 2017 Aug 29;14(1):34. doi: 10.1186/s12989-017-0215-3. Part Fibre Toxicol. 2017. PMID: 28851397 Free PMC article.
-
MicroRNA-335-5p modulates spatial memory and hippocampal synaptic plasticity.Neurobiol Learn Mem. 2017 Mar;139:63-68. doi: 10.1016/j.nlm.2016.12.019. Epub 2016 Dec 27. Neurobiol Learn Mem. 2017. PMID: 28039088
-
BAI1 regulates spatial learning and synaptic plasticity in the hippocampus.J Clin Invest. 2015 Apr;125(4):1497-508. doi: 10.1172/JCI74603. Epub 2015 Mar 9. J Clin Invest. 2015. PMID: 25751059 Free PMC article.
-
Synaptic plasticity and depression: the role of miRNAs dysregulation.Mol Biol Rep. 2022 Oct;49(10):9759-9765. doi: 10.1007/s11033-022-07461-7. Epub 2022 Apr 20. Mol Biol Rep. 2022. PMID: 35441941 Review.
-
miR-140-5p and miR-140-3p: Key Actors in Aging-Related Diseases?Int J Mol Sci. 2022 Sep 28;23(19):11439. doi: 10.3390/ijms231911439. Int J Mol Sci. 2022. PMID: 36232738 Free PMC article. Review.
Cited by
-
The microRNA/TET3/REST axis is required for olfactory globose basal cell proliferation and male behavior.EMBO Rep. 2020 Sep 3;21(9):e49431. doi: 10.15252/embr.201949431. Epub 2020 Jul 17. EMBO Rep. 2020. PMID: 32677323 Free PMC article.
-
Fetal Brain-Derived Exosomal miRNAs from Maternal Blood: Potential Diagnostic Biomarkers for Fetal Alcohol Spectrum Disorders (FASDs).Int J Mol Sci. 2024 May 27;25(11):5826. doi: 10.3390/ijms25115826. Int J Mol Sci. 2024. PMID: 38892014 Free PMC article.
-
miR-9 utilizes precursor pathways in adaptation to alcohol in mouse striatal neurons.Adv Drug Alcohol Res. 2023;3:11323. doi: 10.3389/adar.2023.11323. Epub 2023 Jun 6. Adv Drug Alcohol Res. 2023. PMID: 38116240 Free PMC article.
-
Discovery and Validation of Circulating microRNAs as Biomarkers for Epileptogenesis after Experimental Traumatic Brain Injury-The EPITARGET Cohort.Int J Mol Sci. 2023 Feb 1;24(3):2823. doi: 10.3390/ijms24032823. Int J Mol Sci. 2023. PMID: 36769143 Free PMC article.
-
NF-κB-regulated microRNA-574-5p underlies synaptic and cognitive impairment in response to atmospheric PM2.5 aspiration.Part Fibre Toxicol. 2017 Aug 29;14(1):34. doi: 10.1186/s12989-017-0215-3. Part Fibre Toxicol. 2017. PMID: 28851397 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
