Neutral Sphingomyelinase-2 Deficiency Ameliorates Alzheimer's Disease Pathology and Improves Cognition in the 5XFAD Mouse
- PMID: 27535912
- PMCID: PMC4987436
- DOI: 10.1523/JNEUROSCI.1429-16.2016
Neutral Sphingomyelinase-2 Deficiency Ameliorates Alzheimer's Disease Pathology and Improves Cognition in the 5XFAD Mouse
Abstract
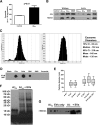
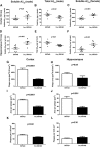
Recent evidence implicates exosomes in the aggregation of Aβ and spreading of tau in Alzheimer's disease. In neural cells, exosome formation can be blocked by inhibition or silencing of neutral sphingomyelinase-2 (nSMase2). We generated genetically nSMase2-deficient 5XFAD mice (fro;5XFAD) to assess AD-related pathology in a mouse model with consistently reduced ceramide generation. We conducted in vitro assays to assess Aβ42 aggregation and glial clearance with and without exosomes isolated by ultracentrifugation and determined exosome-induced amyloid aggregation by particle counting. We analyzed brain exosome content, amyloid plaque formation, neuronal degeneration, sphingolipid, Aβ42 and phospho-tau levels, and memory-related behaviors in 5XFAD versus fro;5XFAD mice using contextual and cued fear conditioning. Astrocyte-derived exosomes accelerated aggregation of Aβ42 and blocked glial clearance of Aβ42 in vitro Aβ42 aggregates were colocalized with extracellular ceramide in vitro using a bifunctional ceramide analog preloaded into exosomes and in vivo using anticeramide IgG, implicating ceramide-enriched exosomes in plaque formation. Compared with 5XFAD mice, the fro;5XFAD mice had reduced brain exosomes, ceramide levels, serum anticeramide IgG, glial activation, total Aβ42 and plaque burden, tau phosphorylation, and improved cognition in a fear-conditioned learning task. Ceramide-enriched exosomes appear to exacerbate AD-related brain pathology by promoting the aggregation of Aβ. Reduction of exosome secretion by nSMase2 loss of function improves pathology and cognition in the 5XFAD mouse model.
Significance statement: We present for the first time evidence, using Alzheimer's disease (AD) model mice deficient in neural exosome secretion due to lack of neutral sphingomyelinase-2 function, that ceramide-enriched exosomes exacerbate AD-related pathologies and cognitive deficits. Our results provide rationale to pursue a means of inhibiting exosome secretion as a potential therapy for individuals at risk for developing AD.
Keywords: 5XFAD; Alzheimer's; ceramide; exosomes; fear conditioning; sphingomyelinase.
Copyright © 2016 the authors 0270-6474/16/368653-15$15.00/0.
Figures





Similar articles
-
Genetic Deletion of Tumor Necrosis Factor-α Attenuates Amyloid-β Production and Decreases Amyloid Plaque Formation and Glial Response in the 5XFAD Model of Alzheimer's Disease.J Alzheimers Dis. 2017;60(1):165-181. doi: 10.3233/JAD-170065. J Alzheimers Dis. 2017. PMID: 28826177
-
Neprilysin deficiency alters the neuropathological and behavioral phenotype in the 5XFAD mouse model of Alzheimer's disease.J Alzheimers Dis. 2015;44(4):1291-302. doi: 10.3233/JAD-142463. J Alzheimers Dis. 2015. PMID: 25408216
-
The 5XFAD Mouse Model of Alzheimer's Disease Exhibits an Age-Dependent Increase in Anti-Ceramide IgG and Exogenous Administration of Ceramide Further Increases Anti-Ceramide Titers and Amyloid Plaque Burden.J Alzheimers Dis. 2015;46(1):55-61. doi: 10.3233/JAD-150088. J Alzheimers Dis. 2015. PMID: 25720409 Free PMC article.
-
Sphingolipid-Enriched Extracellular Vesicles and Alzheimer's Disease: A Decade of Research.J Alzheimers Dis. 2017;60(3):757-768. doi: 10.3233/JAD-160567. J Alzheimers Dis. 2017. PMID: 27662306 Free PMC article. Review.
-
Plasma neuronal exosomes serve as biomarkers of cognitive impairment in HIV infection and Alzheimer's disease.J Neurovirol. 2019 Oct;25(5):702-709. doi: 10.1007/s13365-018-0695-4. Epub 2019 Jan 4. J Neurovirol. 2019. PMID: 30610738 Free PMC article. Review.
Cited by
-
Extracellular Vesicles as an Endocrine Mechanism Connecting Distant Cells.Mol Cells. 2022 Nov 30;45(11):771-780. doi: 10.14348/molcells.2022.0110. Epub 2022 Nov 3. Mol Cells. 2022. PMID: 36380729 Free PMC article. Review.
-
Methylxanthines Induce a Change in the AD/Neurodegeneration-Linked Lipid Profile in Neuroblastoma Cells.Int J Mol Sci. 2022 Feb 18;23(4):2295. doi: 10.3390/ijms23042295. Int J Mol Sci. 2022. PMID: 35216410 Free PMC article.
-
Aberrant early endosome biogenesis mediates complement activation in the retinal pigment epithelium in models of macular degeneration.Proc Natl Acad Sci U S A. 2018 Sep 4;115(36):9014-9019. doi: 10.1073/pnas.1805039115. Epub 2018 Aug 20. Proc Natl Acad Sci U S A. 2018. PMID: 30126999 Free PMC article.
-
Function of ceramide transfer protein for biogenesis and sphingolipid composition of extracellular vesicles.J Extracell Vesicles. 2022 Jun;11(6):e12233. doi: 10.1002/jev2.12233. J Extracell Vesicles. 2022. PMID: 35642450 Free PMC article.
-
Exosomes in the Diseased Brain: First Insights from In vivo Studies.Front Neurosci. 2017 Mar 23;11:142. doi: 10.3389/fnins.2017.00142. eCollection 2017. Front Neurosci. 2017. PMID: 28386213 Free PMC article. Review.
References
-
- An K, Klyubin I, Kim Y, Jung JH, Mably AJ, O'Dowd ST, Lynch T, Kanmert D, Lemere CA, Finan GM, Park JW, Kim TW, Walsh DM, Rowan MJ, Kim JH. Exosomes neutralize synaptic-plasticity-disrupting activity of Abeta assemblies in vivo. Mol Brain. 2013;6:47. doi: 10.1186/1756-6606-6-47. - DOI - PMC - PubMed
-
- Aubin I, Adams CP, Opsahl S, Septier D, Bishop CE, Auge N, Salvayre R, Negre-Salvayre A, Goldberg M, Guénet JL, Poirier C. A deletion in the gene encoding sphingomyelin phosphodiesterase 3 (Smpd3) results in osteogenesis and dentinogenesis imperfecta in the mouse. Nat Genet. 2005;37:803–805. doi: 10.1038/ng1603. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
