New targeted treatments for non-small-cell lung cancer - role of nivolumab
- PMID: 27536062
- PMCID: PMC4975160
- DOI: 10.2147/BTT.S87878
New targeted treatments for non-small-cell lung cancer - role of nivolumab
Abstract
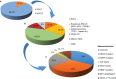
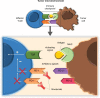
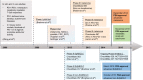
Non-small-cell lung cancer (NSCLC) is often diagnosed at an advanced stage of disease, where it is no longer amenable to curative treatment. During the last decades, the survival has only improved significantly for lung cancer patients who have tumors harboring a driver mutation. Therefore, there is a clear unmet need for effective therapies for patients with no mutation. Immunotherapy has emerged as an effective treatment for different cancer types. Nivolumab, a monoclonal inhibitory antibody against PD-1 receptor, can prolong survival of NSCLC patients, with a manageable toxicity profile. In two Phase III trials, nivolumab was compared to docetaxel in patients with, respectively, squamous (CheckMate 017) and non-squamous NSCLC (CheckMate 057). In both trials, nivolumab significantly reduced the risk of death compared to docetaxel (41% and 27% lower risk of death for squamous and non-squamous NSCLC, respectively). Therefore, nivolumab has been approved in the US and in Europe as second-line treatment for advanced NSCLC. Unfortunately, accurate predictive factors for patient selection are lacking, making it difficult to decide who will benefit and who will not. Currently, there are many ongoing trials that evaluate the efficacy of nivolumab in different settings and in combination with other agents. This paper reviews the present literature about the role of nivolumab in the treatment of NSCLC. Particular attention has been given to efficacy studies, toxicity profile, and current and emerging predictive factors.
Keywords: advanced non-small-cell lung cancer; anti-PD-1; immunotherapy; nivolumab.
Figures
References
-
- Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.2, cancer incidence and mortality worldwide: IARC cancerbase no. 11. Lyon: International Agency for Research on Cancer; 2015. [Accessed January 7, 2016]. Available from: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx.
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. - PubMed
-
- Reck M, Popat S, Reinmuth N, DeRuysscher D, Kerr KM, Peters S. Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(Suppl 3):iii27–iii39. - PubMed
-
- Ettinger DS, Wood DE, Akerley W, et al. NCCN Clinical Practice guidelines in oncology. Non-small cell lung cancer. Version 7. 2015. Available from: http://www.cjcpt.org/files/2015/03-06/2015-NCCN/2015%20NCCN-%E9%9D%9E%E5....
-
- Naidoo J, Drilon A. Molecular diagnostic testing in non-small cell lung cancer. Am J Hematol Oncol. 2014;10(4):4–11.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

