The Alterations of Cortical Volume, Thickness, Surface, and Density in the Intermediate Sporadic Parkinson's Disease from the Han Population of Mainland China
- PMID: 27536237
- PMCID: PMC4971022
- DOI: 10.3389/fnagi.2016.00185
The Alterations of Cortical Volume, Thickness, Surface, and Density in the Intermediate Sporadic Parkinson's Disease from the Han Population of Mainland China
Abstract
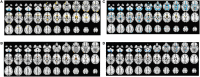
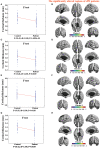
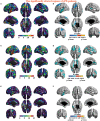
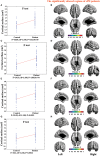
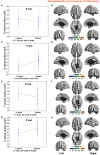
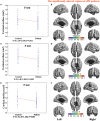
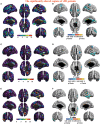
Many symptoms of sporadic Parkinson's disease (sPD) can't be completely explained by the lesion of simple typical extrapyramidal circuit between striatum and substantia nigra. Therefore, we investigated the alteration of cortical volume, thickness, surface, and density in the intermediate sPD from the Han population of Mainland China in order to find the new pathological brain regions associated with the complex clinical manifestations of sPD. The cortical volume, thickness, surface and density were examined using the voxel-based cortical morphometry and corticometry on magnetic resonance image (MRI) in 67 intermediate sPD and 35 controls, the multiple adjusted comparisons analysis of all MRI data were employed to assess the relationships between the cortical morphometric alteration in the specific brain regions and sPD. Results showed that a significantly shrunk volume, thinned thickness and enlarged or reduced surface of cortex in some specific brain regions were closely associated with sPD, but all cortical densities were not different. The majority of morphometric alteration of hemisphere cortex was symmetric, but that in the left hemisphere was more significant. The cortical morphometric alterations in the frontal, temporal, parietal, occipital and limbic lobe, cerebellum, caudate, and thalamus were closely related to the clinical neural dysfunction (Clinical manifestations) of sPD. Our data indicated that the deficits of extensive brain regions involved in the development of sPD, resulted in a series of correspondent complex clinical manifestations in the disease.
Keywords: Han population; Mainland China; cortical morphometry; magnetic resonance image; sporadic Parkinson's disease.
Figures

Similar articles
-
Cortical Structural Connectivity Alterations and Potential Pathogenesis in Mid-Stage Sporadic Parkinson's Disease.Front Aging Neurosci. 2021 May 31;13:650371. doi: 10.3389/fnagi.2021.650371. eCollection 2021. Front Aging Neurosci. 2021. PMID: 34135748 Free PMC article.
-
The cortical thickness correlates of clinical manifestations in the mid-stage sporadic Parkinson's disease.Neurosci Lett. 2016 Oct 28;633:279-289. doi: 10.1016/j.neulet.2016.09.042. Epub 2016 Oct 7. Neurosci Lett. 2016. PMID: 27721206
-
The cortical surface correlates of clinical manifestations in the mid-stage sporadic Parkinson's disease.Neurosci Lett. 2016 Oct 28;633:125-133. doi: 10.1016/j.neulet.2016.09.024. Epub 2016 Sep 17. Neurosci Lett. 2016. PMID: 27651064
-
Patterns of cortical thickness and surface area in early Parkinson's disease.Neuroimage. 2011 Mar 15;55(2):462-7. doi: 10.1016/j.neuroimage.2010.12.043. Epub 2010 Dec 22. Neuroimage. 2011. PMID: 21184830
-
Brain MRI morphometric analysis in Parkinson's disease patients with sleep disturbances.BMC Neurol. 2018 Jun 20;18(1):88. doi: 10.1186/s12883-018-1092-6. BMC Neurol. 2018. PMID: 29925331 Free PMC article.
Cited by
-
Cortical Structural Connectivity Alterations and Potential Pathogenesis in Mid-Stage Sporadic Parkinson's Disease.Front Aging Neurosci. 2021 May 31;13:650371. doi: 10.3389/fnagi.2021.650371. eCollection 2021. Front Aging Neurosci. 2021. PMID: 34135748 Free PMC article.
-
Changes in Cortical Thickness in Patients With Early Parkinson's Disease at Different Hoehn and Yahr Stages.Front Hum Neurosci. 2018 Nov 27;12:469. doi: 10.3389/fnhum.2018.00469. eCollection 2018. Front Hum Neurosci. 2018. PMID: 30542273 Free PMC article.
-
Machine Learning Models for Diagnosis of Parkinson's Disease Using Multiple Structural Magnetic Resonance Imaging Features.Front Aging Neurosci. 2022 Apr 13;14:808520. doi: 10.3389/fnagi.2022.808520. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35493923 Free PMC article.
-
Cortical thickness in Parkinson's disease: a coordinate-based meta-analysis.Aging (Albany NY). 2021 Jan 10;13(3):4007-4023. doi: 10.18632/aging.202368. Epub 2021 Jan 10. Aging (Albany NY). 2021. PMID: 33461168 Free PMC article.
-
Quantitative susceptibility mapping as an indicator of subcortical and limbic iron abnormality in Parkinson's disease with dementia.Neuroimage Clin. 2018 Jul 27;20:365-373. doi: 10.1016/j.nicl.2018.07.028. eCollection 2018. Neuroimage Clin. 2018. PMID: 30128274 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

