Adapting Drug Approval Pathways for Bacteriophage-Based Therapeutics
- PMID: 27536293
- PMCID: PMC4971087
- DOI: 10.3389/fmicb.2016.01209
Adapting Drug Approval Pathways for Bacteriophage-Based Therapeutics
Abstract
The global rise of multi-drug resistant bacteria has resulted in the notion that an "antibiotic apocalypse" is fast approaching. This has led to a number of well publicized calls for global funding initiatives to develop new antibacterial agents. The long clinical history of phage therapy in Eastern Europe, combined with more recent in vitro and in vivo success, demonstrates the potential for whole phage or phage based antibacterial agents. To date, no whole phage or phage derived products are approved for human therapeutic use in the EU or USA. There are at least three reasons for this: (i) phages possess different biological, physical, and pharmacological properties compared to conventional antibiotics. Phages need to replicate in order to achieve a viable antibacterial effect, resulting in complex pharmacodynamics/pharmacokinetics. (ii) The specificity of individual phages requires multiple phages to treat single species infections, often as part of complex cocktails. (iii) The current approval process for antibacterial agents has evolved with the development of chemically based drugs at its core, and is not suitable for phages. Due to similarities with conventional antibiotics, phage derived products such as endolysins are suitable for approval under current processes as biological therapeutic proteins. These criteria render the approval of phages for clinical use theoretically possible but not economically viable. In this review, pitfalls of the current approval process will be discussed for whole phage and phage derived products, in addition to the utilization of alternative approval pathways including adaptive licensing and "Right to try" legislation.
Keywords: adaptive pathways; alternative licensing; bacteriophage; phage therapy; pharmaceutical regulation.
Figures
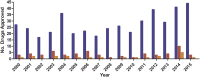
 , Total;
, Total;  , anti-infective;
, anti-infective;  , anti-bacterial.
, anti-bacterial.
Similar articles
-
Engineered Bacteriophage Therapeutics: Rationale, Challenges and Future.BioDrugs. 2021 May;35(3):255-280. doi: 10.1007/s40259-021-00480-z. Epub 2021 Apr 21. BioDrugs. 2021. PMID: 33881767 Free PMC article. Review.
-
Enhancing Whole Phage Therapy and Their Derived Antimicrobial Enzymes through Complex Formulation.Pharmaceuticals (Basel). 2018 Apr 19;11(2):34. doi: 10.3390/ph11020034. Pharmaceuticals (Basel). 2018. PMID: 29671806 Free PMC article. Review.
-
Formulation, stabilisation and encapsulation of bacteriophage for phage therapy.Adv Colloid Interface Sci. 2017 Nov;249:100-133. doi: 10.1016/j.cis.2017.05.014. Epub 2017 May 14. Adv Colloid Interface Sci. 2017. PMID: 28688779 Review.
-
Phage therapy: An alternative to antibiotics in the age of multi-drug resistance.World J Gastrointest Pharmacol Ther. 2017 Aug 6;8(3):162-173. doi: 10.4292/wjgpt.v8.i3.162. World J Gastrointest Pharmacol Ther. 2017. PMID: 28828194 Free PMC article. Review.
-
Viruses versus bacteria-novel approaches to phage therapy as a tool against multidrug-resistant pathogens.J Antimicrob Chemother. 2014 Sep;69(9):2326-36. doi: 10.1093/jac/dku173. Epub 2014 May 28. J Antimicrob Chemother. 2014. PMID: 24872344 Review.
Cited by
-
Implications of Bacteriophage- and Bacteriophage Component-Based Therapies for the Clinical Microbiology Laboratory.J Clin Microbiol. 2019 Jul 26;57(8):e00229-19. doi: 10.1128/JCM.00229-19. Print 2019 Aug. J Clin Microbiol. 2019. PMID: 31092596 Free PMC article. Review.
-
Bacteriophages for Chronic Wound Treatment: from Traditional to Novel Delivery Systems.Viruses. 2020 Feb 20;12(2):235. doi: 10.3390/v12020235. Viruses. 2020. PMID: 32093349 Free PMC article. Review.
-
Chronic Rhinosinusitis and the Evolving Understanding of Microbial Ecology in Chronic Inflammatory Mucosal Disease.Clin Microbiol Rev. 2017 Jan;30(1):321-348. doi: 10.1128/CMR.00060-16. Clin Microbiol Rev. 2017. PMID: 27903594 Free PMC article. Review.
-
Antimicrobial activity of bacteriophage derived triple fusion protein against Staphylococcus aureus.AIMS Microbiol. 2019 Jun 25;5(2):158-175. doi: 10.3934/microbiol.2019.2.158. eCollection 2019. AIMS Microbiol. 2019. PMID: 31384710 Free PMC article.
-
Beyond antibiotics: phage-encoded lysins against Gram-negative pathogens.Front Microbiol. 2023 Sep 8;14:1170418. doi: 10.3389/fmicb.2023.1170418. eCollection 2023. Front Microbiol. 2023. PMID: 37789862 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

