Differential Roles for VviGST1, VviGST3, and VviGST4 in Proanthocyanidin and Anthocyanin Transport in Vitis vinífera
- PMID: 27536314
- PMCID: PMC4971086
- DOI: 10.3389/fpls.2016.01166
Differential Roles for VviGST1, VviGST3, and VviGST4 in Proanthocyanidin and Anthocyanin Transport in Vitis vinífera
Abstract
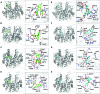
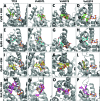
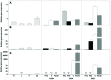
In plant cells, flavonoids are synthesized in the cytosol and then are transported and accumulated in the vacuole. Glutathione S-transferase-mediated transport has been proposed as a mechanism involved in flavonoid transport, however, whether binding of flavonoids to glutathione S-transferase (GST) or their transport is glutathione-dependent is not well understood. Glutathione S-transferases from Vitis vinífera (VviGSTs) have been associated with the transport of anthocyanins, however, their ability to transport other flavonoids such as proanthocyanidins (PAs) has not been established. Following bioinformatics approaches, we analyzed the capability of VviGST1, VviGST3, VviGST4, and Arabidopsis TT19 to bind different flavonoids. Analyses of protein-ligand interactions indicate that these GSTs can bind glutathione and monomers of anthocyanin, PAs and flavonols. A total or partial overlap of the binding sites for glutathione and flavonoids was found in VviGST1, and a similar condition was observed in VviGST3 using anthocyanin and flavonols as ligands, whereas VviGST4 and TT19 have both sites for GSH and flavonoids separated. To validate the bioinformatics predictions, functional complementation assays using the Arabidopsis tt19 mutant were performed. Overexpression of VviGST3 in tt19-1 specifically rescued the dark seed coat phenotype associated to correct PA transport, which correlated with higher binding affinity for PA precursors. VviGST4, originally characterized as an anthocyanin-related GST, complemented both the anthocyanin and PA deposition, resembling the function of TT19. By contrast, VviGST1 only partially rescued the normal seed color. Furthermore the expression pattern of these VviGSTs showed that each of these genes could be associated with the accumulation of different flavonoids in specific tissues during grapevine fruit development. These results provide new insights into GST-mediated PA transport in grapevine and suggest that VviGSTs present different specificities for flavonoid ligands. In addition, our data provide evidence to suggest that GST-mediate flavonoid transport is glutathione-dependent.
Keywords: GST; anthocyanins; flavonoid transport; glutathione S-transferase; grapevine; proanthocyanidins.
Figures


Similar articles
-
The Arabidopsis tt19-4 mutant differentially accumulates proanthocyanidin and anthocyanin through a 3' amino acid substitution in glutathione S-transferase.Plant Cell Environ. 2011 Mar;34(3):374-88. doi: 10.1111/j.1365-3040.2010.02249.x. Epub 2010 Dec 1. Plant Cell Environ. 2011. PMID: 21054438
-
TRANSPARENT TESTA 19 is involved in the accumulation of both anthocyanins and proanthocyanidins in Arabidopsis.Plant J. 2004 Jan;37(1):104-14. doi: 10.1046/j.1365-313x.2003.01943.x. Plant J. 2004. PMID: 14675436
-
Metabolic profiling and cytological analysis of proanthocyanidins in immature seeds of Arabidopsis thaliana flavonoid accumulation mutants.Plant J. 2010 May 1;62(4):549-59. doi: 10.1111/j.1365-313X.2010.04174.x. Epub 2010 Feb 18. Plant J. 2010. PMID: 20180920
-
Biochemistry and Molecular Basis of Intracellular Flavonoid Transport in Plants.Plants (Basel). 2022 Apr 1;11(7):963. doi: 10.3390/plants11070963. Plants (Basel). 2022. PMID: 35406945 Free PMC article. Review.
-
Plant flavonoids--biosynthesis, transport and involvement in stress responses.Int J Mol Sci. 2013 Jul 17;14(7):14950-73. doi: 10.3390/ijms140714950. Int J Mol Sci. 2013. PMID: 23867610 Free PMC article. Review.
Cited by
-
Three Camellia sinensis glutathione S-transferases are involved in the storage of anthocyanins, flavonols, and proanthocyanidins.Planta. 2019 Oct;250(4):1163-1175. doi: 10.1007/s00425-019-03206-2. Epub 2019 Jun 8. Planta. 2019. PMID: 31177387
-
Zebularine, a DNA Methylation Inhibitor, Activates Anthocyanin Accumulation in Grapevine Cells.Genes (Basel). 2022 Jul 15;13(7):1256. doi: 10.3390/genes13071256. Genes (Basel). 2022. PMID: 35886036 Free PMC article.
-
Proanthocyanidins and Where to Find Them: A Meta-Analytic Approach to Investigate Their Chemistry, Biosynthesis, Distribution, and Effect on Human Health.Antioxidants (Basel). 2021 Jul 30;10(8):1229. doi: 10.3390/antiox10081229. Antioxidants (Basel). 2021. PMID: 34439477 Free PMC article. Review.
-
Genome-Wide Identification of Glutathione S-Transferase Genes in Eggplant (Solanum melongena L.) Reveals Their Potential Role in Anthocyanin Accumulation on the Fruit Peel.Int J Mol Sci. 2024 Apr 11;25(8):4260. doi: 10.3390/ijms25084260. Int J Mol Sci. 2024. PMID: 38673847 Free PMC article.
-
Natural variations in the Cis-elements of GhRPRS1 contributing to petal colour diversity in cotton.Plant Biotechnol J. 2024 Dec;22(12):3473-3488. doi: 10.1111/pbi.14468. Epub 2024 Sep 16. Plant Biotechnol J. 2024. PMID: 39283921 Free PMC article.
References
-
- Baxter I. R., Young J. C., Armstrong G., Foster N., Bogenschutz N., Cordova T., et al. (2005). A plasma membrane H+-ATPase is required for the formation of proanthocyanidins in the seed coat endothelium of Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 102 2649–2654. 10.1073/pnas.0406377102 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

