Acid reducing agents to neonates - lack of evidence and guidelines
- PMID: 27536424
- PMCID: PMC4980723
- DOI: 10.3109/21556660.2012.655816
Acid reducing agents to neonates - lack of evidence and guidelines
Abstract
Objective: The aim of this retrospective study was to investigate the clinical practice, i.e. the frequency of use and the treatment strategies, for acid reducing drugs to neonates in a Swedish hospital.
Methods: Retrospective reviews of charts and interviews with nurses at the neonatal wards of Karolinska University Hospital were performed to identify difficulties that might occur with drug administration. All patients admitted over a 2-month period were included. Main outcome measure were the number of patients treated with acid reducing drugs and the dosages.
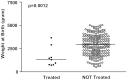
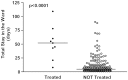
Results: Nine out of 215 patients (4.2%) received an acid reducing drug. Patients treated with acid reducing drugs had significantly lower birth weight, lower gestational age and longer duration of hospitalization. Eight of the patients were treated with omeprazole. One of these patients started treatment with omeprazole but continued later on with ranitidine. One patient was exclusively treated with ranitidine. The doses of omeprazole (intravenous or oral administration) were within the range 0.16-1.26 mg/kg/day.
Conclusions: A wide variation in treatment regimens of acid reducing drugs is given to newborn infants. The percentage of treated children was much lower than earlier reports from the US and UK. No conclusions can be drawn as to whether the doses and dosing intervals used give sufficient acid suppression, since the effect of the therapy was not recorded. The present study is only retrospective and data are not truly comparable with other studies. Further studies are therefore warranted to evaluate effective doses and pharmacokinetics of acid reducing drugs in newborn infants.
Keywords: Histamine2 receptor antagonists; Neonates; Pharmaceutical preparations; Proton pump inhibitors.
Figures

Similar articles
-
Concomitant Administration of a Histamine2 Receptor Antagonist and Proton Pump Inhibitor Enhances Gastric Acid Suppression.Pharmacotherapy. 2015 Dec;35(12):1124-9. doi: 10.1002/phar.1665. Epub 2015 Dec 1. Pharmacotherapy. 2015. PMID: 26621819 Clinical Trial.
-
Pharmacokinetic optimisation in the treatment of gastro-oesophageal reflux disease.Clin Pharmacokinet. 1996 Nov;31(5):386-406. doi: 10.2165/00003088-199631050-00005. Clin Pharmacokinet. 1996. PMID: 9118586 Review.
-
Effect of gastric pH on erlotinib pharmacokinetics in healthy individuals: omeprazole and ranitidine.Anticancer Drugs. 2015 Jun;26(5):565-72. doi: 10.1097/CAD.0000000000000212. Anticancer Drugs. 2015. PMID: 25643050 Clinical Trial.
-
Evaluation of gastric pH and guaiac measurements in neonates receiving acid suppression therapy during extracorporeal membrane oxygenation.Pharmacotherapy. 2004 Sep;24(9):1130-6. doi: 10.1592/phco.24.13.1130.38085. Pharmacotherapy. 2004. PMID: 15460173
-
Omeprazole. A review of its use in Helicobacter pylori infection, gastro-oesophageal reflux disease and peptic ulcers induced by nonsteroidal anti-inflammatory drugs.Drugs. 1998 Sep;56(3):447-86. doi: 10.2165/00003495-199856030-00012. Drugs. 1998. PMID: 9777317 Review.
References
-
- James LP, Farrar HC, Palmer K, et al. Gastrointestinal drugs. In Yaffe SJ, Aranda JV. Neonatal and Paediatric Pharmacology- Therapeutics Principles in Practice, 3rd edn. Philadelphia: Lippincott Williams & Wilkins, 2005
-
- Grahnquist L, Ruuska T, Finkel Y. Early development of human gastric H, K- adenosine triphosphatase. J Pediar Gastroenterol Nutr 2000;30:533-7 - PubMed
-
- Kelly EJ, Brownlee KG, Newell SJ. Gastric secretory function in the developing human stomach. Early Hum Dev 1992;31:163-6 - PubMed
-
- Kaijser M, Akre O, Cnattingius S, et al. Preterm birth, low birth weight, and risk for esophageal adenocarcinoma. Gastroenterology 2005;128:607-9 - PubMed
-
- Akre O, Forssell L, Kaijser M, et al. Perinatal risk factors for cancer of the esophagus and gastric cardia: a nested case-control study. Cancer Epidemiol Biomarkers Prev 2006;15:867-71 - PubMed
LinkOut - more resources
Full Text Sources
