Trial of pranlukast inhibitory effect for cedar exposure using an OHIO chamber
- PMID: 27536428
- PMCID: PMC4980729
- DOI: 10.3109/21556660.2012.703630
Trial of pranlukast inhibitory effect for cedar exposure using an OHIO chamber
Abstract
Objective: In practical guidelines for management of allergic rhinitis in Japan, pranlukast is a leukotriene receptor antagonist recommended for the treatment of pollinosis. However, the effect of pranlukast on nasal symptoms for cedar pollinosis has not been thoroughly investigated. The aim of this study is to examine this effect in a double-blind controlled crossover study using a pollen challenge chamber (the OHIO Chamber) developed in Japan.
Research design and methods: A total of 39 patients with cedar pollinosis were targeted. The subjects were exposed to a specific amount of cedar pollen (8000/m(3)) in the OHIO Chamber during the non-cedar pollen season. Efficacy of pranlukast for the treatment of artificially induced nasal symptoms was compared with that of a placebo using the crossover method. Pranlukast was administered orally for 3 days, after dinner on the day before cedar pollen exposure, after breakfast and after dinner on the day of cedar pollen exposure, and after breakfast on the following day. Pollen testing was carried out twice, with a 1-week wash-out interval.
Clinical trial registration: The University Hospital Medical Information Network in Japan (UMIN), number UMIN000001282.
Main outcome measures: The effect of pranlukast was evaluated using self-rating of nasal symptoms by the subjects.
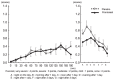
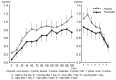
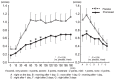
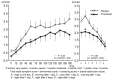
Results: All 39 subjects demonstrated a positive skin reaction to cedar pollen by a positive CAP-RAST score (class 2 or higher) within the last 3 years, and experienced aggravated congestion during the cedar pollen season for more than 2 years. Nasal congestion was inhibited significantly in the pranlukast group compared to the placebo group during cedar pollen exposure. Furthermore, pranlukast significantly inhibited nasal congestion compared to the placebo on the day after exposure and on the following day.
Conclusions: The effect of pranlukast on cedar pollinosis indicates immediate action, and such an effect could take place continuously after cedar pollen exposure. These results demonstrate that pranlukast is effective for the relief of congestion due to pollinosis.
Keywords: Cedar pollinosis; Crossover trial; Nasal symptoms; OHIO chamber; Pranlukast.
Figures
Similar articles
-
Placebo-controlled study with OHIO chamber of prophylactic pranlukast for children with Japanese cedar pollinosis: TOPIC-J III study.J Drug Assess. 2014 Sep 3;3(1):51-9. doi: 10.3109/21556660.2014.960969. eCollection 2014. J Drug Assess. 2014. PMID: 27536454 Free PMC article.
-
Beneficial effects of leukotriene receptor antagonists in the prevention of cedar pollinosis in a community setting.J Investig Allergol Clin Immunol. 2009;19(3):195-203. J Investig Allergol Clin Immunol. 2009. PMID: 19610262
-
Delay of onset of symptoms of Japanese cedar pollinosis by treatment with a leukotriene receptor antagonist.Allergol Int. 2011 Dec;60(4):483-9. doi: 10.2332/allergolint.10-OA-0285. Epub 2011 Jul 25. Allergol Int. 2011. PMID: 21778814 Clinical Trial.
-
Evaluation of Montelukast for the Treatment of Children With Japanese Cedar Pollinosis Using an Artificial Exposure Chamber (OHIO Chamber).Allergy Rhinol (Providence). 2018 Jul 13;9:2152656718783599. doi: 10.1177/2152656718783599. eCollection 2018 Jan-Dec. Allergy Rhinol (Providence). 2018. PMID: 30027002 Free PMC article.
-
[Studies on the experimental allergic rhinitis induced by Japanese cedar pollen--role of cysteinyl leukotrienes in nasal allergic symptoms].Yakugaku Zasshi. 2003 Jan;123(1):1-8. doi: 10.1248/yakushi.123.1. Yakugaku Zasshi. 2003. PMID: 12607939 Review. Japanese.
Cited by
-
Placebo-controlled study with OHIO chamber of prophylactic pranlukast for children with Japanese cedar pollinosis: TOPIC-J III study.J Drug Assess. 2014 Sep 3;3(1):51-9. doi: 10.3109/21556660.2014.960969. eCollection 2014. J Drug Assess. 2014. PMID: 27536454 Free PMC article.
-
International Consensus Statement on Allergy and Rhinology: Allergic Rhinitis.Int Forum Allergy Rhinol. 2018 Feb;8(2):108-352. doi: 10.1002/alr.22073. Int Forum Allergy Rhinol. 2018. PMID: 29438602 Free PMC article.
-
A randomized controlled phase II clinical trial comparing ONO-4053, a novel DP1 antagonist, with a leukotriene receptor antagonist pranlukast in patients with seasonal allergic rhinitis.Allergy. 2017 Oct;72(10):1565-1575. doi: 10.1111/all.13174. Epub 2017 May 16. Allergy. 2017. PMID: 28378369 Free PMC article. Clinical Trial.
References
-
- Worldwide variation in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis and atopic eczema: ISAAC The International Study of Asthma and Allergies in Childhood (ISAAC) Steering Committee. Lancet 1998;351:1225-32 - PubMed
-
- Okuda M. Epidemiology of Japanese cedar pollinosis throughout Japan. Ann Allergy Asthma Immunol 2003;91:288-96 - PubMed
-
- Sakashita M, Hirota T, Harada M, et al. Prevalence of allergic rhinitis and sensitization to common aeroallergens in a Japanese population. Int Arch Allergy Immunol 2010;151:255-61 - PubMed
-
- Yonetomi Y, Fujita M, Nakagawa N, et al. Preclinical pharmacology of pranlukast. Clin Exp Allergy Rev 2001;1:210-17
-
- Tamaoki J, Kondo M, Sakai N, et al. Leukotriene antagonist prevents exacerbation of asthma during reduction of high-dose inhaled corticosteroid. Am J Respir Crit Care Med 1997;155:1235-40 - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
