Aliskiren/amlodipine as a single-pill combination in hypertensive patients: subgroup analysis of elderly patients, with metabolic risk factors or high body mass index
- PMID: 27536431
- PMCID: PMC4937657
- DOI: 10.3109/21556660.2012.762367
Aliskiren/amlodipine as a single-pill combination in hypertensive patients: subgroup analysis of elderly patients, with metabolic risk factors or high body mass index
Abstract
Aims: Blood pressure (BP) reduction in hypertensive patients is more difficult to achieve in the elderly or in the presence of comorbidities. We aimed to investigate the efficacy of the single-pill combination (SPC) aliskiren/amlodipine in hypertensive elderly patients, patients with high body mass index (BMI), with at least one metabolic risk factor, and/or type 2 diabetes mellitus (DM).
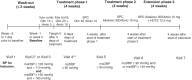
Methods: In an open-label non-randomized study, patients not adequately controlled by previous treatment with the SPC olmesarten 40/amlodipine 10 (phase 1) were switched to the SPC aliskiren 300/amlodipine 10 (phase 2). The present post-hoc analysis investigated BP reduction in phase 2 in the named subgroups. The EudraCT identifier was 2009-016693-33, ClinicalTrials.gov identifier NCT01113047.
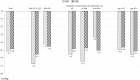
Results: Of the 187 patients not adequately controlled in phase 1 and thus treated with the SPC aliskiren 300/amlodipine 10 in phase 2, 69 were of advanced age (≥65 years), 74 or 89 were overweight or obese (BMI 25.0-29.9 kg/m(2) or ≥30 kg/m(2), respectively), 91 had metabolic risk factors (without DM) and 41 had DM. At the beginning of phase 2, depending on the subgroup, baseline SBP was 168-169 mmHg and DBP 103-104 mmHg. After 4 weeks of treatment with aliskiren 300/amlodipine 10, SBP/DBP was lowered by -5.1/-4.8 mmHg in the total cohort, by -5.5/-5.1 mmHg in elderly patients, by -6.7/-5.5 in overweight and by -4.2/-4.5 mmHg in obese patients, by -6.4/-4.7 mmHg in patients with metabolic risk factors without DM, and by -3.3/-5.0 mmHg in DM patients. Limitations include low sample size, limited treatment duration and the fact that the post-hoc defined groups were not mutually exclusive.
Conclusions: In this study reflecting clinical practice, the aliskiren/amlodipine combination achieved effective BP reduction in elderly patients or with metabolic comorbidities, including DM that might be more difficult to treat. This consistent BP lowering pattern facilitates everyday care of patients who receive aliskiren/amlodipine.
Keywords: Aliskiren; Amlodipine; Arterial hypertension; Combination therapy; Diabetes mellitus; Elderly; Metabolic risk factors; Olmesartan.
Figures
References
-
- Wong ND Dede J Chow VH et al. Global cardiovascular risk associated with hypertension and extent of treatment and control according to risk group. Am J Hypertens 2012;25:561-7 - PubMed
-
- Dahlof B Sever PS Poulter NR et al. Prevention of cardiovascular events with an antihypertensive regimen of amlodipine adding perindopril as required versus atenolol adding bendroflumethiazide as required, in the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm (ASCOT-BPLA): a multicentre randomised controlled trial. Lancet 2005;366:895-906 - PubMed
-
- Julius S Kjeldsen SE Weber M et al. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet 2004;363:2022-31 - PubMed
-
- Ruschitzka F. Evidence for improvement in survival with antihypertensive combination treatment. J Hypertens 2011;29(Suppl 1):S9-14 - PubMed
-
- Mancia G De Backer G Dominiczak A et al. 2007 Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J 2007;28:1462-536 - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
