Delivery characteristics of a low-resistance dry-powder inhaler used to deliver the long-acting muscarinic antagonist glycopyrronium
- PMID: 27536432
- PMCID: PMC4937662
- DOI: 10.3109/21556660.2013.766197
Delivery characteristics of a low-resistance dry-powder inhaler used to deliver the long-acting muscarinic antagonist glycopyrronium
Abstract
Objectives: The long-acting muscarinic antagonist (LAMA) glycopyrronium (NVA237) has recently been approved as a once-daily treatment for COPD. The objectives of this study were to determine the dose delivery characteristics of glycopyrronium and compare them with those of the LAMA tiotropium, both delivered by their respective capsule-based dry-powder inhalers (DPIs).
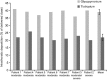
Research design and methods: Seven inhalation profiles derived from patients with moderate and severe COPD were reproduced to determine the aerodynamic particle size distribution of glycopyrronium delivered by the Breezhaler device, a low-resistance DPI†. Theoretical respiratory tract deposition was estimated using a semi-empirical model for healthy lungs. These results were compared with those of tiotropium delivered by the high-resistance HandiHaler‡ device obtained in a previous study using the same set of inhalation profiles. Study limitations are that fine particle fraction (FPF) and particle size are generated by the inhalers are not a direct measure of lung deposition, and the bronchodilator effect of inhaled drugs does not depend solely upon the percentage of the total dose that reaches the lung.
Results: The mean FPF (≤4.7 µm) was 42.6% of the nominal dose (which refers to the content of the capsule) for glycopyrronium and 9.8% for tiotropium while the mass median aerodynamic diameter (MMAD) was 2.8 µm and 3.9 µm for glycopyrronium and tiotropium, respectively. The mean estimated intrathoracic drug deposition as a percentage of the mean dose delivered to the Next Generation Impactor was 39% for glycopyrronium and 22% for tiotropium.
Conclusions: The glycopyrronium capsule-based DPI delivered a higher FPF and greater and more consistent intrathoracic deposition irrespective of age and disease severity compared to the tiotropium capsule-based DPI, suggesting that it may be suitable for use by patients with a wide range of COPD severities.
Keywords: COPD; Dry-powder inhaler; Glycopyrronium; Tiotropium.
Figures


Similar articles
-
Lung deposition of inhaled once-daily long-acting muscarinic antagonists via standard jet nebulizer or dry powder inhaler, measured using functional respiratory imaging, in patients with chronic obstructive pulmonary disease.Ther Adv Respir Dis. 2022 Jan-Dec;16:17534666221077561. doi: 10.1177/17534666221077561. Ther Adv Respir Dis. 2022. PMID: 35234085 Free PMC article.
-
Once-daily glycopyrronium via the Breezhaler® device for the treatment of COPD: pharmacological and clinical profile.Expert Rev Clin Pharmacol. 2013 Sep;6(5):503-17. doi: 10.1586/17512433.2013.828419. Epub 2013 Aug 24. Expert Rev Clin Pharmacol. 2013. PMID: 23971870 Review.
-
In Vitro Effect of Different Airflow Rates on the Aerosol Properties of Nebulized Glycopyrrolate in the eFlow® Closed System and Tiotropium Delivered in the HandiHaler®.Pulm Ther. 2020 Dec;6(2):289-301. doi: 10.1007/s41030-020-00125-6. Epub 2020 Aug 18. Pulm Ther. 2020. PMID: 32809156 Free PMC article.
-
Indacaterol/Glycopyrronium/Mometasone: A Review in Asthma.Drugs. 2021 Apr;81(6):709-719. doi: 10.1007/s40265-021-01518-w. Epub 2021 Apr 19. Drugs. 2021. PMID: 33871819 Review.
-
The clinical relevance of dry powder inhaler performance for drug delivery.Respir Med. 2014 Aug;108(8):1195-203. doi: 10.1016/j.rmed.2014.05.009. Epub 2014 May 24. Respir Med. 2014. PMID: 24929253
Cited by
-
Inhibitory effects of glycopyrronium, formoterol, and budesonide on coronavirus HCoV-229E replication and cytokine production by primary cultures of human nasal and tracheal epithelial cells.Respir Investig. 2020 May;58(3):155-168. doi: 10.1016/j.resinv.2019.12.005. Epub 2020 Feb 21. Respir Investig. 2020. PMID: 32094077 Free PMC article.
-
Lung Deposition and Inspiratory Flow Rate in Patients with Chronic Obstructive Pulmonary Disease Using Different Inhalation Devices: A Systematic Literature Review and Expert Opinion.Int J Chron Obstruct Pulmon Dis. 2021 Apr 19;16:1021-1033. doi: 10.2147/COPD.S297980. eCollection 2021. Int J Chron Obstruct Pulmon Dis. 2021. PMID: 33907390 Free PMC article.
-
Biophysical model to predict lung delivery from a dual bronchodilator dry-powder inhaler.Int J Pharm X. 2019 May 30;1:100018. doi: 10.1016/j.ijpx.2019.100018. eCollection 2019 Dec. Int J Pharm X. 2019. PMID: 31517283 Free PMC article.
-
The Repeatability of Inspiration Performance Through Different Inhalers in Patients with Chronic Obstructive Pulmonary Disease and Control Volunteers.J Aerosol Med Pulm Drug Deliv. 2020 Oct;33(5):271-281. doi: 10.1089/jamp.2020.1594. Epub 2020 May 28. J Aerosol Med Pulm Drug Deliv. 2020. PMID: 32460588 Free PMC article.
-
Comparison of peak inspiratory flow rate via the Breezhaler®, Ellipta® and HandiHaler® dry powder inhalers in patients with moderate to very severe COPD: a randomized cross-over trial.BMC Pulm Med. 2018 Jun 14;18(1):100. doi: 10.1186/s12890-018-0662-0. BMC Pulm Med. 2018. PMID: 29898702 Free PMC article. Clinical Trial.
References
-
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the diagnosis, management and prevention of COPD. Updated December 2011. www goldcopd org 2011 [cited 2012 Aug 30]
-
- Geller DE. Comparing clinical features of the nebulizer, metered-dose inhaler, and dry powder inhaler. Respir Care 2005;50:1313-21 - PubMed
-
- Girodet PO Raherison C Abouelfath A et al. [Real-life use of inhaler devices for chronic obstructive pulmonary disease in primary care]. Therapie 2003;58:499-504 - PubMed
-
- Laube BL Janssens HM de Jongh FH et al. What the pulmonary specialist should know about the new inhalation therapies. Eur Respir J 2011;37:1308-31 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
