Bioequivalence study of two subcutaneous formulations of dalteparin: randomized, single-dose, two-sequence, two-period, cross-over study in healthy volunteers
- PMID: 27536434
- PMCID: PMC4937650
- DOI: 10.3109/21556660.2013.781504
Bioequivalence study of two subcutaneous formulations of dalteparin: randomized, single-dose, two-sequence, two-period, cross-over study in healthy volunteers
Abstract
Objective: This study assessed relative bioavailability of a new subcutaneous formulation, test (T) (dalteparin sodium 95000 IU/3.8 mL) with the branded product (R) in healthy subjects to meet the regulatory requirements of bioequivalence in the US.
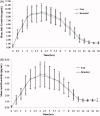
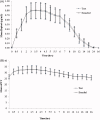
Methods: This was an open label, randomized, single dose, two-sequence, two-period cross-over study under fasting conditions. A total of 88 healthy adult volunteers were randomized to either of the treatment arms (T or R) separated by a washout period of 7 days. Pharmacodynamic surrogates, namely anti-Xa and anti-IIa activity, heparin clotting assay (heptest), and activated partial thromboplastin time (aPTT) were used as a tool to establish bioequivalence between these two formulations. Blood samples were collected up to 36 h post-dose to characterize the primary pharmacokinetic parameters A max, AUC0- t , and AUC0-∞ for anti-Xa and anti-IIa and heptest; parameters (Δt )max and AU(Δt ) for aPTT.
Results: For anti-Xa activity, the means (SD) of A max (IU/mL) were 1.34 (0.25) [range = 0.59-2.03] and 1.39 (0.35) [range = 0.65-2.69]; AUC0- t (IU•h/mL) values were 11.4 (2.76) [range = 2.89-19.5] and 12.1 (2.87) [range = 2.52-21.30]; AUC0 - ∞ (IU•h/mL) values were 13.1 (3.59) [range = 3.15-28.2] and 14.5 (4.97) [range = 2.79-36.1] for test and branded formulations, respectively. For anti-IIa activity, the means (SD) of A max (IU/mL) were 0.34 (0.12) [range = 0.14-0.72] and 0.34 (0.13) [range = 0.11-0.84]; AUC0- t (IU•h/mL) values were 2.05 (0.72) [range = 0.61-4.69] and 2.11 (0.76) [range = 0.84-4.80]; AUC0 - ∞ (IU•h/mL) values were 2.47 (0.80) [range = 0.76-6.29] and 2.61 (0.86) [range = 1.31-5.36], for test and branded formulations, respectively. The 90% CI for all the primary pharmacokinetic parameters of all the pharmacodynamic surrogates tested met the regulatory bioequivalence criterion of 80.00-125.00%.
Conclusion: The test product met the US regulatory criteria of bioequivalence relative to the branded product in this single dose bioequivalence study. Study limitations include open-label single dose design.
Keywords: Bioavailability; Bioequivalence; Dalteparin; Subcutaneous.
Figures
Similar articles
-
Bioequivalence of generic and branded subcutaneous enoxaparin: a single-dose, randomized-sequence, open-label, two-period crossover study in healthy Chinese male subjects.Clin Ther. 2009 Jul;31(7):1559-67. doi: 10.1016/j.clinthera.2009.07.017. Clin Ther. 2009. PMID: 19695405 Clinical Trial.
-
Bioavailability and pharmacokinetic comparison between generic and branded azithromycin capsule: a randomized, double-blind, 2-way crossover in healthy male Thai volunteers.Clin Ther. 2007 Apr;29(4):703-10. doi: 10.1016/j.clinthera.2007.04.010. Clin Ther. 2007. PMID: 17617293 Clinical Trial.
-
Bioavailability of omeprazole 20 mg capsules containing omeprazole 22.5% enteric coated pellets versus a reference product in healthy Bangladeshi male subjects: an open-label, single-dose, randomized-sequence, two-way crossover study.Int J Clin Pharmacol Ther. 2011 Dec;49(12):778-86. doi: 10.5414/cp201569. Int J Clin Pharmacol Ther. 2011. PMID: 22122821 Clinical Trial.
-
Bioequivalence of a biosimilar enoxaparin (Cloti-Xa™) and its innovator (Clexane® ): A single-dose, randomized, double-blind, two-period, two-treatment, two-sequence, crossover, balanced study in healthy human subjects.Pharmacol Res Perspect. 2022 Aug;10(4):e00979. doi: 10.1002/prp2.979. Pharmacol Res Perspect. 2022. PMID: 35762448 Free PMC article. Review.
-
Study on requirements of bioequivalence for registration of pharmaceutical products in USA, Europe and Canada.Saudi Pharm J. 2014 Nov;22(5):391-402. doi: 10.1016/j.jsps.2013.05.001. Epub 2013 May 31. Saudi Pharm J. 2014. PMID: 25473327 Free PMC article. Review.
References
-
- Lip GYH, Chin BSP, Blann AD. Cancer and the prothrombotic state. Lancet Oncol 2002;3:27-34 - PubMed
-
- Hirsh J Bauer KA Donati MB et al. Parenteral anticoagulants: American college of chest physicians evidence-based clinical practice guidelines. 8th edn. Chest 2008;133:141S-59 - PubMed
-
- Hirsh J, Levine MN. Low molecular weight heparin. Blood 1992;79:1-17 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous