Effects of dalfampridine and its metabolites on cloned human potassium channels Kv 1.1, Kv 1.2, and Kv 1.4 expressed in human embryonic kidney cells
- PMID: 27536438
- PMCID: PMC4937658
- DOI: 10.3109/21556660.2013.791623
Effects of dalfampridine and its metabolites on cloned human potassium channels Kv 1.1, Kv 1.2, and Kv 1.4 expressed in human embryonic kidney cells
Abstract
Background: Dalfampridine (4-aminopyridine; 4-AP) is a potassium channel blocker that has been available in the United States as a treatment to improve walking in patients with multiple sclerosis. 4-AP is well-characterized in vitro with regard to inhibition of neuronal potassium channels, but the potential contribution of its metabolites to clinical activity has not been determined. This study evaluated the concentration-response of 4-AP and its two primary metabolites, 3-hydroxy-4-aminopyridine and 3-hydroxy-4-aminopyridine sulfate, for inhibition of the potassium channels Kv 1.1, Kv 1.2, and Kv 1.4, which are considered candidates for mediating effects of 4-AP on action potential conduction because of their presence in axonal membranes.
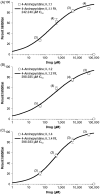
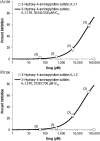
Methods: Stable transfection of cDNA for Kv 1.1, Kv 1.2, and Kv 1.4 was performed into HEK293 cells, and colonies of cells containing each channel were selected and maintained under appropriate cell culture conditions. Electrophysiological measurements were performed using a patch-clamp technique in at least three cells for each concentration (50, 500, 5000, and 50,000 μM) of 4-AP and the two metabolites, with each cell acting as its own control. Concentration-response curves were constructed for 4-AP and each metabolite. Data were analyzed using nonlinear least-squares fit, and concentrations inhibiting the channels by 50% (IC50) were estimated.
Results: 4-AP induced similar concentration-dependent inhibition profiles of all three potassium channels, resulting in a narrow range of IC50 values across channels (242 µM to 399 µM). Across the three channels, the IC50 values of 3-hydroxy-4-aminopyridine and 3-hydroxy-4-aminopyridine sulfate were 1-2 orders of magnitude higher (less potent) than those of 4-AP.
Conclusions: 3-Hydroxy-4-aminopyridine and 3-hydroxy-4-aminopyridine sulfate demonstrated low in vitro potency for Kv 1.1, Kv 1.2, and Kv 1.4 inhibition, suggesting that these metabolites are unlikely to contribute to the positive pharmacodynamic effects of 4-AP. A limitation of this study is that while the metabolites were substantially less active at these representative potassium channels in vitro, the untested possibility exists that they may be active at one or more of the many other channel types that occur in vivo.
Keywords: 4-Aminopyridine; Dalfampridine; Metabolites; Potassium channels.
Figures

References
-
- Ampyra® (dalfampridine) extended release tablets [package insert]. Ardsley, NY: Acorda Therapeutics, Inc.; 2011
-
- Coetzee WA Amarillo Y Chiu J et al. Molecular diversity of K+ channels. Ann N Y Acad Sci 1999;868:233-85 - PubMed
-
- Judge SI, Bever CT., Jr Potassium channel blockers in multiple sclerosis: neuronal Kv channels and effects of symptomatic treatment. Pharmacol Ther 2006;111:224-59 - PubMed
-
- Rudy B. Voltage-gated K+ channels. In: Kew J, Davies C, eds. Ion Channels, From Structure to Function. Oxford: Oxford University Press, 2010:21-57
-
- Goodman AD Brown TR Edwards KR et al. A phase 3 trial of extended release oral dalfampridine in multiple sclerosis. Ann Neurol 2010;68:494-502 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
