Efficacy of fluticasone furoate nasal spray and levocetirizine in patients with Japanese cedar pollinosis subjected to an artificial exposure chamber
- PMID: 27536443
- PMCID: PMC4937659
- DOI: 10.3109/21556660.2013.829070
Efficacy of fluticasone furoate nasal spray and levocetirizine in patients with Japanese cedar pollinosis subjected to an artificial exposure chamber
Abstract
Objective: This study investigated the clinical efficacy of a combination therapy of levocetirizine (LCTZ) and fluticasone furoate nasal spray (FFNS), compared with LCTZ monotherapy, for the suppression of seasonal allergic rhinitis (SAR) symptoms induced in an artificial exposure chamber.
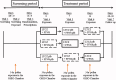
Methods: This study was a single-center, placebo-controlled, randomized, 3-way cross-over comparative study performed in 42 Japanese cedar pollinosis patients. These subjects received (1) LCTZ plus FFNS (combination group), (2) LCTZ plus FFNS placebo (monotherapy group), or (3) LCTZ placebo plus FFNS placebo (placebo group) once on the night prior to exposure, with a 1-week washout period between exposures. Nasal (sneezing, rhinorrhea, nasal congestion, and itchy nose) and ocular (eye itching and tearing) symptoms were recorded every 15 min, and the number of sneezes, nose blowing events, and the amount of nasal secretions were measured during exposure. The primary end-point was the cumulative incidence of SAR symptoms during exposure and the 'ime to occurrence of symptoms'. The secondary end-points were the total nasal symptom score, the ocular symptom score, the amount of nasal discharge, and the number of sneezes and nose blowing events.
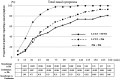
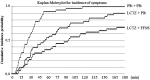
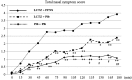
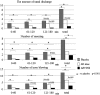
Results: At all the measurement points, the lowest cumulative incidences for the nasal symptoms were observed in the combination group, followed by the monotherapy and placebo groups. All the subjects in the placebo group developed nasal symptoms within 2 h after pollen exposure, while three and eight subjects in the monotherapy and combination groups, respectively, did not develop any nasal symptoms during exposure. In addition, combination therapy delayed the onset of symptoms.
Conclusions: The results demonstrated that combination therapy with FFNS and LCTZ significantly suppressed the induced SAR symptoms and delayed the onset of symptoms compared with LCTZ monotherapy and placebo. Although the conditions of the allergen challenge study using an exposure chamber are different from those in real life, combination therapy with FF and LCTZ was confirmed to be an effective treatment for SAR.
Keywords: Artificial exposure chamber; Combination therapy; Fluticasone furoate nasal spray; Japanese cedar pollinosis; Levocetirizine.
Figures
Similar articles
-
Effectiveness of fluticasone furoate 110 microg once daily in the treatment of nasal and ocular symptoms of seasonal allergic rhinitis in adults and adolescents sensitized to mountain cedar pollen.Curr Med Res Opin. 2009 Jun;25(6):1393-401. doi: 10.1185/03007990902890512. Curr Med Res Opin. 2009. PMID: 19419338 Clinical Trial.
-
Comparison of fluticasone furoate and fluticasone propionate for the treatment of Japanese cedar pollinosis.Allergy Asthma Proc. 2009 Jan-Feb;30(1):84-94. doi: 10.2500/aap.2009.30.3182. Epub 2008 Dec 4. Allergy Asthma Proc. 2009. PMID: 19061537 Clinical Trial.
-
An integrated analysis of the efficacy of fluticasone furoate nasal spray on individual nasal and ocular symptoms of seasonal allergic rhinitis.Allergy Asthma Proc. 2010 Nov-Dec;31(6):483-92. doi: 10.2500/aap.2010.31.3397. Allergy Asthma Proc. 2010. PMID: 21708060
-
Fluticasone furoate nasal spray consistently and significantly improves both the nasal and ocular symptoms of seasonal allergic rhinitis: a review of the clinical data.Expert Opin Pharmacother. 2008 Oct;9(15):2707-15. doi: 10.1517/14656566.9.15.2707. Expert Opin Pharmacother. 2008. PMID: 18803457 Review.
-
Evaluation of Intranasal Corticosteroid Sensory Attributes and Patient Preference for Fluticasone Furoate for the Treatment of Allergic Rhinitis.Clin Ther. 2019 Aug;41(8):1589-1596. doi: 10.1016/j.clinthera.2019.05.017. Epub 2019 Aug 8. Clin Ther. 2019. PMID: 31402060 Review.
References
-
- Asher MI Monterfort S Björkstén B et al. the ISAAC Phase Three Study Group . Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC phases one and three repeat multicountry cross-sectional surveys. Lancet 2006;368:733-43 - PubMed
-
- Okubo K Kurono Y Fujieda S et al. Japanese guideline for allergic rhinitis. Allergol Int 2011;60:171-89 - PubMed
-
- Meltzer EO Nathan R Derebery J et al. Sleep, quality of life, and productivity impact of nasal symptoms in the United States: finding from the burden of rhinitis in America survey. Allergy Asthma Proc 2009;30:244-54 - PubMed
-
- Meltzer EO Blaiss MS Derebery MJ et al. Burden of allergic rhinitis: results from the pediatric allergies in American survey. J Allergy Clin Immunol 2009;124(3 Suppl):S43-70 - PubMed
-
- Bousquet J Khaltaev N Cruz AA et al. Allergic rhinitis and its impact on asthma (ARIA) 2008. Allergy 2008;63(86 Suppl):8-160 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
