Pharmacodynamic evaluation of intragastric pH and implications for famotidine dosing in the prophylaxis of non-steroidal anti-inflammatory drug induced gastropathy-a proof of concept analysis
- PMID: 27536450
- PMCID: PMC4937634
- DOI: 10.3109/21556660.2014.895371
Pharmacodynamic evaluation of intragastric pH and implications for famotidine dosing in the prophylaxis of non-steroidal anti-inflammatory drug induced gastropathy-a proof of concept analysis
Abstract
Objective: Famotidine given at a dose of 80 mg/day is effective in preventing NSAID-induced gastropathy. The aim of this proof of concept study was to compare twice a day (BID) vs 3-times a day (TID) administration of this total dose of famotidine on intragastric pH in healthy volunteers.
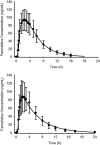
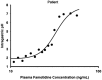
Research design and methods: Two analyses were undertaken: (1) a 13 subject controlled cross-over 24-h intragastric pH evaluation of the BID and TID administration of 80 mg/day of famotidine, as well as measures for drug accumulation over 5 days (EudraCT, number 2006-002930-39); and (2) a pharmacokinetic (PK)/pharmacodynamic (PD) model which predicted steady-state famotidine plasma concentrations and pH of the two regimens.
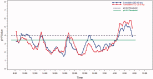
Results: For the cross-over study, gastric pH was above 3.5 for a mean of 20 min longer for TID dosing compared to BID dosing on Day 1. On Day 5, the mean time above this threshold was higher with the BID regimen by ∼25 min. For pH 4, subjects' gastric pH was above this pH value for a mean of 25 min longer for TID dosing compared to BID dosing on Day 1. For Day 5, the pH was above 4 for ∼45 min longer with the TID regimen as compared with the BID regimen. The mean 24-h gastric pH values when taken in the upright position trended higher for the TID dosing period compared to the BID regimen on Day 1. The steady-state simulation model indicated that, following TID dosing, intragastric pH will be above 3 for 24 h vs 16 h for the BID regimen. There was no evidence for plasma accumulation of famotidine with TID dosing as compared to BID dosing from either analysis.
Conclusion: The data indicate that overall more time is spent above the acidic threshold pH values when 80 mg/day of famotidine is administered TID vs BID. Key limitations included small study size with a short duration and lack of a baseline examination, but was compensated for by the cross-over and PK/PD modeling design. Although most of the comparisons in this proof of concept study were not statistically significant these results have important implications for future research on gastric acid lowering agents used for the prevention of NSAID-induced gastropathy.
Keywords: Famotidine; Gastropathy; Intragastric; NSAID; Prophylaxis; pH.
Figures



Similar articles
-
Low-dose famotidine and effervescent cimetidine in healthy subjects: a placebo-controlled overnight pH study.Aliment Pharmacol Ther. 1998 May;12(5):469-74. doi: 10.1046/j.1365-2036.1998.00323.x. Aliment Pharmacol Ther. 1998. PMID: 9663728 Clinical Trial.
-
Pharmacokinetics of gabapentin after a single day and at steady state following the administration of gastric-retentive- extended-release and immediate-release tablets: a randomized, open-label, multiple-dose, three-way crossover, exploratory study in healthy subjects.Clin Ther. 2008 May;30(5):909-16. doi: 10.1016/j.clinthera.2008.05.008. Clin Ther. 2008. PMID: 18555937 Clinical Trial.
-
Application of PK/PD modeling and simulation to dosing regimen optimization of high-dose human regular U-500 insulin.J Diabetes Sci Technol. 2014 Jul;8(4):821-9. doi: 10.1177/1932296814532326. Epub 2014 May 12. J Diabetes Sci Technol. 2014. PMID: 24876428 Free PMC article. Clinical Trial.
-
Short and long-acting oral nitrates for stable angina pectoris.Cardiovasc Drugs Ther. 1994 Aug;8(4):611-23. doi: 10.1007/BF00877415. Cardiovasc Drugs Ther. 1994. PMID: 7848896 Review.
-
The First-in-Class Potassium-Competitive Acid Blocker, Vonoprazan Fumarate: Pharmacokinetic and Pharmacodynamic Considerations.Clin Pharmacokinet. 2016 Apr;55(4):409-18. doi: 10.1007/s40262-015-0326-7. Clin Pharmacokinet. 2016. PMID: 26369775 Review.
References
-
- Food and Drug Administration (FDA), Center for Drug Evaluation and Research (CDER), Gastrointestinal Drugs Advisor Committee (GIDAC). Advisory Meeting, Hilton Washington DC/North, Gaithersburg, VA, November 4, 2010
-
- Masso Gonzalez EL, Patrignani P, Tacconelli S, et al. Variability among nonsteroidal anti-inflammatory drugs in risk of upper gastrointestinal bleeding. Arthritis Rheum 2010;62:1592–601 - PubMed
-
- Chang CH, Chen HC, Lin JW, et al. Risk of hospitalization for upper gastrointestinal adverse events associated with nonsteroidal anti-inflammatory drugs: a nationwide case-crossover study in Taiwan. Pharmacoepidemiol Drug Saf 2011;20:763–71 - PubMed
-
- Niculescu L, Li C, Huang J, et al. Pooled analysis of GI tolerability of 21 randomized controlled trials of celecoxib and nonselective NSAIDs. Curr Med Res Opin 2009;25:729–40 - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
