Targeting p97 to Disrupt Protein Homeostasis in Cancer
- PMID: 27536557
- PMCID: PMC4971439
- DOI: 10.3389/fonc.2016.00181
Targeting p97 to Disrupt Protein Homeostasis in Cancer
Abstract
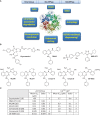
Cancer cells are addicted to numerous non-oncogenic traits that enable them to thrive. Proteotoxic stress is one such non-oncogenic trait that is experienced by all tumor cells owing to increased genomic abnormalities and the resulting synthesis and accumulation of non-stoichiometric amounts of cellular proteins. This imbalance in the amounts of proteins ultimately culminates in proteotoxic stress. p97, or valosin-containing protein (VCP), is an ATPase whose function is essential to restore protein homeostasis in the cells. Working in concert with the ubiquitin proteasome system, p97 promotes the retrotranslocation from cellular organelles and/or degradation of misfolded proteins. Consequently, p97 inhibition has emerged as a novel therapeutic target in cancer cells, especially those that have a highly secretory phenotype. This review summarizes our current understanding of the function of p97 in maintaining protein homeostasis and its inhibition with small molecule inhibitors as an emerging strategy to target cancer cells.
Keywords: CDC48; ER stress; ERAD; VCP; cancer; p97 inhibitors; proteotoxic stress.
Figures
References
-
- Frohlich KU, Fries HW, Rudiger M, Erdmann R, Botstein D, Mecke D. Yeast cell cycle protein CDC48p shows full-length homology to the mammalian protein VCP and is a member of a protein family involved in secretion, peroxisome formation, and gene expression. J Cell Biol (1991) 114(3):443–53.10.1083/jcb.114.3.443 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

