A New Method to Stabilize C-Kit Expression in Reparative Cardiac Mesenchymal Cells
- PMID: 27536657
- PMCID: PMC4971111
- DOI: 10.3389/fcell.2016.00078
A New Method to Stabilize C-Kit Expression in Reparative Cardiac Mesenchymal Cells
Abstract
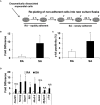
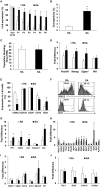
Cell therapy improves cardiac function. Few cells have been investigated more extensively or consistently shown to be more effective than c-kit sorted cells; however, c-kit expression is easily lost during passage. Here, our primary goal was to develop an improved method to isolate c-kit(pos) cells and maintain c-kit expression after passaging. Cardiac mesenchymal cells (CMCs) from wild-type mice were selected by polystyrene adherence properties. CMCs adhering within the first hours are referred to as rapidly adherent (RA); CMCs adhering subsequently are dubbed slowly adherent (SA). Both RA and SA CMCs were c-kit sorted. SA CMCs maintained significantly higher c-kit expression than RA cells; SA CMCs also had higher expression endothelial markers. We subsequently tested the relative efficacy of SA vs. RA CMCs in the setting of post-infarct adoptive transfer. Two days after coronary occlusion, vehicle, RA CMCs, or SA CMCs were delivered percutaneously with echocardiographic guidance. SA CMCs, but not RA CMCs, significantly improved cardiac function compared to vehicle treatment. Although the mechanism remains to be elucidated, the more pronounced endothelial phenotype of the SA CMCs coupled with the finding of increased vascular density suggest a potential pro-vasculogenic action. This new method of isolating CMCs better preserves c-kit expression during passage. SA CMCs, but not RA CMCs, were effective in reducing cardiac dysfunction. Although c-kit expression was maintained, it is unclear whether maintenance of c-kit expression per se was responsible for improved function, or whether the differential adherence property itself confers a reparative phenotype independently of c-kit.
Keywords: c-kit; cell therapy; heart failure; mesenchymal cell; myocardial repair.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

