Overview of the Pathogenesis of ANCA-Associated Vasculitis
- PMID: 27536680
- PMCID: PMC4934824
- DOI: 10.1159/000442323
Overview of the Pathogenesis of ANCA-Associated Vasculitis
Abstract
Background: Antineutrophil cytoplasmic autoantibodies (ANCA) are associated with a spectrum of necrotizing vasculitis including granulomatosis with polyangiitis, microscopic polyangiitis, eosinophilic granulomatosis with polyangiitis, and renal-limited necrotizing and crescentic glomerulonephritis. Clinical observations and in vitro and in vivo experimental evidence strongly indicate that ANCA are pathogenic.
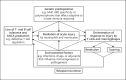
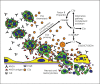
Summary: The etiology and pathogenesis of ANCA-associated vasculitis (AAV) are multifactorial, with contributions from genetic factors, environmental exposures, infections, characteristics of the innate and adaptive immune system, and the intensity and duration of the injury. Acute vascular inflammation is induced when resting neutrophils that have ANCA autoantigens sequestered in cytoplasmic granules are exposed to priming factors - for example, cytokines induced by infection or phlogogenic factors released by complement activation - that cause the release of ANCA antigens on the surface of neutrophils and in the microenvironment around the neutrophils. ANCA bind to these ANCA antigens, which activates neutrophils by Fcγ receptor engagement and F(ab')2 binding at the neutrophil cell surface. ANCA-activated neutrophils release factors that activate the alternative complement pathway, which generates C5a, a chemoattractant for neutrophils; C5a also primes the arriving neutrophils for activation by ANCA. Activated neutrophils adhere to and penetrate vessel walls, and they release toxic oxygen radicals and destructive enzymes that cause apoptosis and necrosis of the neutrophils as well as of the adjacent vessel wall cells and matrix.
Key messages: Patients with active AAV have ongoing asynchronous onsets of countless acute lesions, with each lesion evolving through stereotypical phases within 1 or 2 weeks. Induction of remission results in termination of new waves of acute lesions and allows all lesions to progress to scarring or resolution.
Keywords: ANCA; Antineutrophil cytoplasmic autoantibodies; Autoantibodies; Polyangiitis; Vasculitis.
Figures

References
-
- Jennette JC, Falk RJ, Andrassy K, Bacon PA, Churg J, Gross WL, Hagen EC, Hoffman GS, Hunder GG, Kallenberg CGM, McCluskey RT, Sinico RA, Rees AJ, van Es LA, Waldherr R, Wiik A. Nomenclature of systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum. 1994;37:187–192. - PubMed
-
- Jennette JC, Falk RJ. Small vessel vasculitis. N Engl J Med. 1997;337:1512–1523. - PubMed
-
- Jennette JC, Falk RJ. The role of pathology in the diagnosis of systemic vasculitis. Clin Exp Rheumatol. 2007;25(suppl 44):52–56. - PubMed
-
- Eisenberger U, Fakhouri F, Vanhille P, Beaufils H, Mahr A, Guillevin L, Lesavre P, Noël LH. ANCA-negative pauci-immune renal vasculitis: histology and outcome. Nephrol Dial Transplant. 2005;20:1392–1399. - PubMed
-
- Falk RJ, Jennette JC. Anti-neutrophil cytoplasmic autoantibodies with specificity for myeloperoxidase in patients with systemic vasculitis and idiopathic necrotizing and crescentic glomerulonephritis. N Engl J Med. 1988;318:1651–1657. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

