Datasets of a novel bivalent single chain antibody constructed by overlapping oligonucleotide annealing method targeting human CD123
- PMID: 27536714
- PMCID: PMC4976644
- DOI: 10.1016/j.dib.2016.07.014
Datasets of a novel bivalent single chain antibody constructed by overlapping oligonucleotide annealing method targeting human CD123
Abstract
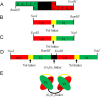
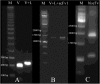
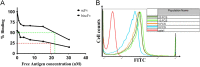
Current therapies for acute myeloid leukemia (AML), are associated with high relapse rates. Hence, development of new therapeutic strategies is crucial to circumvent this problem. Bivalent antibody technology has been used to engineer novel antibody fragments with increased avidity, by assembling two scFv in a single molecule. Here, we present accompanying data from construction and characterization experiments of a biscFv antibody targeting CD123, the most important biomarker of leukemic cancer stem cells which play a key role in relapsed AML after chemotherapy. Data in this article are related to the research paper "Development of a novel engineered antibody targeting human CD123" Moradi-Kalbolandi S. et al. (2016) [1].
Figures


Similar articles
-
Development of a novel engineered antibody targeting human CD123.Anal Biochem. 2016 Oct 15;511:27-30. doi: 10.1016/j.ab.2016.04.017. Epub 2016 May 5. Anal Biochem. 2016. PMID: 27156812
-
Soluble Expression and Characterization of a New scFv Directed to Human CD123.Appl Biochem Biotechnol. 2016 Apr;178(7):1390-406. doi: 10.1007/s12010-015-1954-y. Epub 2016 Jan 9. Appl Biochem Biotechnol. 2016. PMID: 26749295
-
Antileukemia Efficacy and Mechanisms of Action of SL-101, a Novel Anti-CD123 Antibody Conjugate, in Acute Myeloid Leukemia.Clin Cancer Res. 2017 Jul 1;23(13):3385-3395. doi: 10.1158/1078-0432.CCR-16-1904. Epub 2017 Jan 17. Clin Cancer Res. 2017. PMID: 28096272 Free PMC article.
-
CD123: A Novel Biomarker for Diagnosis and Treatment of Leukemia.Cardiovasc Hematol Disord Drug Targets. 2019;19(3):195-204. doi: 10.2174/1871529X19666190627100613. Cardiovasc Hematol Disord Drug Targets. 2019. PMID: 31244444 Review.
-
CD123 as a Therapeutic Target in the Treatment of Hematological Malignancies.Cancers (Basel). 2019 Sep 12;11(9):1358. doi: 10.3390/cancers11091358. Cancers (Basel). 2019. PMID: 31547472 Free PMC article. Review.
References
-
- Moradi-Kalbolandi S. Development of a novel engineered antibody targeting human CD123. Anal. Biochem. 2016 - PubMed
-
- Moradi-Kalbolandi S. Soluble expression and characterization of a new scfv directed to human CD123. Appl. Biochem. Biotechnol. 2016:1–17. - PubMed
-
- Sambrook J., Fritsch E.F., Maniatis T. Vol. 2. Cold Spring Harbor Laboratory Press; New York: 1989. (Molecular cloning).
-
- Rath S., Stanley C., Steward M. An inhibition enzyme immunoassay for estimating relative antibody affinity and affinity heterogeneity. J. Immunol. Methods. 1988;106(2):245–249. - PubMed
-
- Lee C.V., Sidhu S.S., Fuh G. Bivalent antibody phage display mimics natural immunoglobulin. J. Immunol. Methods. 2004;284(1):119–132. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

