Thiacalix[4]arene: New protection for metal nanoclusters
- PMID: 27536724
- PMCID: PMC4982710
- DOI: 10.1126/sciadv.1600323
Thiacalix[4]arene: New protection for metal nanoclusters
Abstract
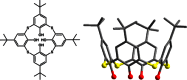
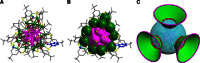
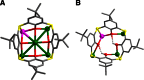
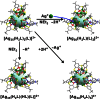
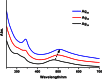
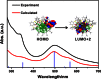
Surface organic ligands are critical for the formation and properties of atomically precise metal nanoclusters. In contrast to the conventionally used protective ligands such as thiolates and phosphines, thiacalix[4]arene has been used in the synthesis of a silver nanocluster, [Ag35(H2L)2(L)(C≡CBu(t))16](SbF6)3, (H4L, p-tert-butylthiacalix[4]-arene). This is the first structurally determined calixarene-protected metal nanocluster. The chelating and macrocyclic effects make the thiacalix[4]arene a rigid shell that protects the silver core. Upon addition or removal of one silver atom, the Ag35 cluster can be transformed to Ag36 or Ag34 species, and the optical properties are changed accordingly. The successful use of thiacalixarene in the synthesis of well-defined silver nanoclusters suggests a bright future for metal nanoclusters protected by macrocyclic ligands.
Keywords: Calixarene; alkynyl ligand; coordination mode; crystal structure; ligand-protected metal nanocluster; silver nanocluster.
Figures



Similar articles
-
All-Calixarene-Protected Silver Nanocluster with All Silver Atoms in a Face-Centered Cubic Arrangement.J Am Chem Soc. 2024 Sep 11;146(36):25101-25107. doi: 10.1021/jacs.4c08094. Epub 2024 Aug 28. J Am Chem Soc. 2024. PMID: 39196903
-
The stability enhancement factor beyond eight-electron shell closure in thiacalix[4]arene-protected silver clusters.Chem Sci. 2019 Feb 4;10(11):3360-3365. doi: 10.1039/c8sc03756f. eCollection 2019 Mar 21. Chem Sci. 2019. PMID: 30996924 Free PMC article.
-
An Ultrastable 155-Nuclei Silver Nanocluster Protected by Thiacalix[4]arene and Cyclohexanethiol for Photothermal Conversion.Angew Chem Int Ed Engl. 2022 Aug 1;61(31):e202206742. doi: 10.1002/anie.202206742. Epub 2022 Jun 15. Angew Chem Int Ed Engl. 2022. PMID: 35589617
-
Advancements in Atomically Precise Nanocluster Protected by Thiacalix[4]arene.Adv Mater. 2024 Nov;36(45):e2410054. doi: 10.1002/adma.202410054. Epub 2024 Sep 3. Adv Mater. 2024. PMID: 39226533 Review.
-
Nitrogen Donor Protection for Atomically Precise Metal Nanoclusters.Chemistry. 2022 Apr 27;28(24):e202104445. doi: 10.1002/chem.202104445. Epub 2022 Mar 8. Chemistry. 2022. PMID: 35218267 Review.
Cited by
-
Achieving stable photoluminescence by double thiacalix[4]arene-capping: the lanthanide-oxo cluster core matters.RSC Adv. 2022 Oct 12;12(45):29151-29161. doi: 10.1039/d2ra04942b. eCollection 2022 Oct 11. RSC Adv. 2022. PMID: 36320769 Free PMC article.
-
Regulating the assembly and expansion of the silver cluster from the Ag37 to Ag46 nanowheel driven by heteroanions.Chem Sci. 2022 Dec 26;14(5):1138-1144. doi: 10.1039/d2sc06436g. eCollection 2023 Feb 1. Chem Sci. 2022. PMID: 36756341 Free PMC article.
-
Vertex-Shared Linear Superatomic Molecules: Stepping Stones to Novel Materials Composed of Noble Metal Clusters.Small Sci. 2023 Apr 24;3(5):2300024. doi: 10.1002/smsc.202300024. eCollection 2023 May. Small Sci. 2023. PMID: 40213321 Free PMC article.
-
Revealing isoelectronic size conversion dynamics of metal nanoclusters by a noncrystallization approach.Nat Commun. 2018 May 17;9(1):1979. doi: 10.1038/s41467-018-04410-6. Nat Commun. 2018. PMID: 29773785 Free PMC article.
-
Distance makes a difference in crystalline photoluminescence.Nat Commun. 2020 Nov 4;11(1):5572. doi: 10.1038/s41467-020-19377-6. Nat Commun. 2020. PMID: 33149132 Free PMC article.
References
-
- Häkkinen H., The gold–sulfur interface at the nanoscale. Nat. Chem. 4, 443–455 (2012). - PubMed
-
- Parker J. F., Fields-Zinna C. A., Murry R. W., The story of a monodisperse gold nanoparticle: Au25L18. Acc. Chem. Res. 43, 1289–1296 (2010). - PubMed
-
- Wilcoxon J. P., Abrams B. L., Synthesis, structure and properties of metal nanoclusters. Chem. Soc. Rev. 35, 1162–1194 (2006). - PubMed
-
- Yamazoe S., Koyasu K., Tsukuda T., Nonscalable oxidation catalysis of gold clusters. Acc. Chem. Res. 47, 816–824 (2014). - PubMed
-
- Jin R., Atomically precise metal nanoclusters: Stable sizes and optical properties. Nanoscale 7, 1549–1565 (2015). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

