Heart-resident CCR2+ macrophages promote neutrophil extravasation through TLR9/MyD88/CXCL5 signaling
- PMID: 27536731
- PMCID: PMC4985028
- DOI: 10.1172/jci.insight.87315
Heart-resident CCR2+ macrophages promote neutrophil extravasation through TLR9/MyD88/CXCL5 signaling
Abstract
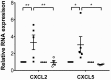
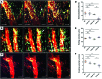
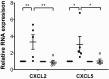
It is well established that maladaptive innate immune responses to sterile tissue injury represent a fundamental mechanism of disease pathogenesis. In the context of cardiac ischemia reperfusion injury, neutrophils enter inflamed heart tissue, where they play an important role in potentiating tissue damage and contributing to contractile dysfunction. The precise mechanisms that govern how neutrophils are recruited to and enter the injured heart are incompletely understood. Using a model of cardiac transplant-mediated ischemia reperfusion injury and intravital 2-photon imaging of beating mouse hearts, we determined that tissue-resident CCR2+ monocyte-derived macrophages are essential mediators of neutrophil recruitment into ischemic myocardial tissue. Our studies revealed that neutrophil extravasation is mediated by a TLR9/MyD88/CXCL5 pathway. Intravital 2-photon imaging demonstrated that CXCL2 and CXCL5 play critical and nonredundant roles in guiding neutrophil adhesion and crawling, respectively. Together, these findings uncover a specific role for a tissue-resident monocyte-derived macrophage subset in sterile tissue inflammation and support the evolving concept that macrophage ontogeny is an important determinant of function. Furthermore, our results provide the framework for targeting of cell-specific signaling pathways in myocardial ischemia reperfusion injury.
Figures





References
-
- El-Sawy T, Belperio JA, Strieter RM, Remick DG, Fairchild RL. Inhibition of polymorphonuclear leukocyte-mediated graft damage synergizes with short-term costimulatory blockade to prevent cardiac allograft rejection. Circulation. 2005;112(3):320–331. doi: 10.1161/CIRCULATIONAHA.104.516708. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

