FOLH1/GCPII is elevated in IBD patients, and its inhibition ameliorates murine IBD abnormalities
- PMID: 27536732
- PMCID: PMC4985244
- DOI: 10.1172/jci.insight.88634
FOLH1/GCPII is elevated in IBD patients, and its inhibition ameliorates murine IBD abnormalities
Abstract
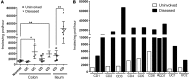
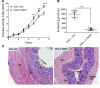
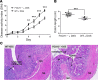
Recent gene-profiling analyses showed significant upregulation of the folate hydrolase (FOLH1) gene in the affected intestinal mucosa of patients with inflammatory bowel disease (IBD). The FOLH1 gene encodes a type II transmembrane glycoprotein termed glutamate carboxypeptidase II (GCPII). To establish that the previously reported increased gene expression was functional, we quantified the glutamate carboxypeptidase enzymatic activity in 31 surgical specimens and report a robust 2.8- to 41-fold increase in enzymatic activity in the affected intestinal mucosa of IBD patients compared with an uninvolved area in the same patients or intestinal mucosa from healthy controls. Using a human-to-mouse approach, we next showed a similar enzymatic increase in two well-validated IBD murine models and evaluated the therapeutic effect of the potent FOLH1/ GCPII inhibitor 2-phosphonomethyl pentanedioic acid (2-PMPA) (IC50 = 300 pM). In the dextran sodium sulfate (DSS) colitis model, 2-PMPA inhibited the GCPII activity in the colonic mucosa by over 90% and substantially reduced the disease activity. The significance of the target was confirmed in FOLH1-/- mice who exhibited resistance to DSS treatment. In the murine IL-10-/- model of spontaneous colitis, daily 2-PMPA treatment also significantly reduced both macroscopic and microscopic disease severity. These results provide the first evidence of FOLH1/GCPII enzymatic inhibition as a therapeutic option for IBD.
Figures


Similar articles
-
Looking for Drugs in All the Wrong Places: Use of GCPII Inhibitors Outside the Brain.Neurochem Res. 2020 Jun;45(6):1256-1267. doi: 10.1007/s11064-019-02909-y. Epub 2019 Nov 20. Neurochem Res. 2020. PMID: 31749072 Free PMC article. Review.
-
Local enema treatment to inhibit FOLH1/GCPII as a novel therapy for inflammatory bowel disease.J Control Release. 2017 Oct 10;263:132-138. doi: 10.1016/j.jconrel.2017.01.036. Epub 2017 Jan 31. J Control Release. 2017. PMID: 28159515 Free PMC article.
-
Deletion of the glutamate carboxypeptidase II gene in mice reveals a second enzyme activity that hydrolyzes N-acetylaspartylglutamate.J Neurochem. 2002 Oct;83(1):20-9. doi: 10.1046/j.1471-4159.2002.01117.x. J Neurochem. 2002. PMID: 12358725
-
Targeting glutamate carboxypeptidase II in IBD.Adv Pharmacol. 2024;101:265-285. doi: 10.1016/bs.apha.2024.10.001. Epub 2024 Oct 15. Adv Pharmacol. 2024. PMID: 39521603 Review.
-
Spontaneous Loss-of-Function Dock2 Mutation Alters Murine Colitis Sensitivity and Is a Confounding Variable in Inflammatory Bowel Disease Research.Crohns Colitis 360. 2019 Oct;1(3):otz030. doi: 10.1093/crocol/otz030. Epub 2019 Sep 26. Crohns Colitis 360. 2019. PMID: 39624652 Free PMC article.
Cited by
-
D-DOPA Is a Potent, Orally Bioavailable, Allosteric Inhibitor of Glutamate Carboxypeptidase II.Pharmaceutics. 2022 Sep 23;14(10):2018. doi: 10.3390/pharmaceutics14102018. Pharmaceutics. 2022. PMID: 36297453 Free PMC article.
-
Structural and computational basis for potent inhibition of glutamate carboxypeptidase II by carbamate-based inhibitors.Bioorg Med Chem. 2019 Jan 15;27(2):255-264. doi: 10.1016/j.bmc.2018.11.022. Epub 2018 Nov 14. Bioorg Med Chem. 2019. PMID: 30552009 Free PMC article.
-
Looking for Drugs in All the Wrong Places: Use of GCPII Inhibitors Outside the Brain.Neurochem Res. 2020 Jun;45(6):1256-1267. doi: 10.1007/s11064-019-02909-y. Epub 2019 Nov 20. Neurochem Res. 2020. PMID: 31749072 Free PMC article. Review.
-
Multiomic Analysis of the Gut Microbiome in Psoriasis Reveals Distinct Host‒Microbe Associations.JID Innov. 2022 Mar 10;2(3):100115. doi: 10.1016/j.xjidi.2022.100115. eCollection 2022 May. JID Innov. 2022. PMID: 35757783 Free PMC article.
-
Solid extraintestinal malignancies in patients with inflammatory bowel disease.World J Gastrointest Oncol. 2021 Dec 15;13(12):1956-1980. doi: 10.4251/wjgo.v13.i12.1956. World J Gastrointest Oncol. 2021. PMID: 35070035 Free PMC article. Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

