Modulation of Immune Responses by Extracellular Vesicles From Retinal Pigment Epithelium
- PMID: 27537259
- PMCID: PMC4991022
- DOI: 10.1167/iovs.15-18353
Modulation of Immune Responses by Extracellular Vesicles From Retinal Pigment Epithelium
Abstract
Purpose: Extracellular vesicles (EV), such as exosomes, are important mediators of intercellular communication and have been implicated in modulation of the immune system. We investigated if EV released from retinal pigment epithelium (RPE) modulate immune responses in vitro.
Methods: Extracellular vesicles were isolated from ARPE-19 cultures stimulated or not with the inflammatory cytokines IL-1β, IFN-γ, and TNF-α. Isolated EV were characterized by nanoparticle flow and Western blot analyses. Retinal pigment epithelium-derived EV were cultured with human peripheral blood mononuclear cells, which were assayed for T-cell proliferation by 3H-thymidine incorporation. Retinal pigment epithelium-derived EV were also independently cultured with enriched lymphocytes or monocytes. Cell phenotype and cell death were evaluated by flow cytometric analysis. Cytokine levels were assayed in culture supernatants by multiplex bead analysis.
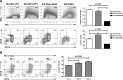
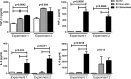
Results: The concentration of ARPE-derived EV from cytokine-stimulated cultures was slightly higher than from nonstimulated cultures. The size of EV was approximately 100 nm in both groups. Extracellular vesicles from both nonstimulated and cytokine-stimulated ARPE-19 significantly inhibited T-cell proliferation without affecting T-cell viability. Culture of EV from nonstimulated ARPE-19 with undifferentiated human monocytes induced an immunoregulatory phenotype with a significantly higher percentage of CD14++CD16+ monocytes and upregulation of TGF-β1. Culture of EV from cytokine-stimulated ARPE-19 cells with human monocytes induced upregulation of several proinflammatory cytokines and monocyte death.
Conclusions: Retinal pigment epithelium cells constitutively secrete EV in the size range of exosomes, with increased release from RPE cells stimulated with inflammatory cytokines. Extracellular vesicles from both nonstimulated and cytokine-stimulated RPE inhibited T-cell stimulation. Extracellular vesicles from nonstimulated ARPE-19 cells promoted an immunoregulatory CD14++CD16+ phenotype in human monocytes, while EV from cytokine-stimulated ARPE-19 cells caused human monocyte death. These findings suggest that RPE cells use EV to induce a downregulatory immune environment under homeostatic conditions. In an inflammatory milieu, RPE-derived EV may mitigate a potentially harmful inflammatory response through killing of monocytes.
Figures


Comment in
-
Extracellular vesicles: important players in immune homeostasis.Ann Transl Med. 2017 May;5(Suppl 1):S16. doi: 10.21037/atm.2017.03.76. Ann Transl Med. 2017. PMID: 28567398 Free PMC article. No abstract available.
Similar articles
-
Extracellular vesicles from regenerative human cardiac cells act as potent immune modulators by priming monocytes.J Nanobiotechnology. 2019 May 27;17(1):72. doi: 10.1186/s12951-019-0504-0. J Nanobiotechnology. 2019. PMID: 31133024 Free PMC article.
-
Green tea polyphenol epigallocatechin-3-gallate attenuates TNF-α-induced intercellular adhesion molecule-1 expression and monocyte adhesion to retinal pigment epithelial cells.Am J Chin Med. 2015;43(1):103-19. doi: 10.1142/S0192415X1550007X. Epub 2015 Feb 2. Am J Chin Med. 2015. PMID: 25644976
-
Extracellular Soluble Membranes from Retinal Pigment Epithelial Cells Mediate Apoptosis in Macrophages.Cells. 2021 May 13;10(5):1193. doi: 10.3390/cells10051193. Cells. 2021. PMID: 34068205 Free PMC article.
-
Proteomics of Retinal Extracellular Vesicles: A Review into an Unexplored Mechanism in Retinal Health and AMD Pathogenesis.Adv Exp Med Biol. 2023;1415:87-94. doi: 10.1007/978-3-031-27681-1_14. Adv Exp Med Biol. 2023. PMID: 37440019 Review.
-
Polarized Exosome Release from the Retinal Pigmented Epithelium.Adv Exp Med Biol. 2018;1074:539-544. doi: 10.1007/978-3-319-75402-4_65. Adv Exp Med Biol. 2018. PMID: 29721985 Review.
Cited by
-
Exploring the utility of extracellular vesicles in ameliorating viral infection-associated inflammation, cytokine storm and tissue damage.Transl Oncol. 2021 Jul;14(7):101095. doi: 10.1016/j.tranon.2021.101095. Epub 2021 Apr 19. Transl Oncol. 2021. PMID: 33887552 Free PMC article. Review.
-
Stem Cell-Derived Extracellular Vesicles as a Potential Therapeutic Tool for Eye Diseases: From Benchtop to Bedside.Adv Exp Med Biol. 2023;1410:127-143. doi: 10.1007/5584_2022_754. Adv Exp Med Biol. 2023. PMID: 36525172
-
The Limbal Niche and Regenerative Strategies.Vision (Basel). 2021 Sep 22;5(4):43. doi: 10.3390/vision5040043. Vision (Basel). 2021. PMID: 34698278 Free PMC article. Review.
-
Mesenchymal stem cells-derived exosomes ameliorate blue light stimulation in retinal pigment epithelium cells and retinal laser injury by VEGF-dependent mechanism.Int J Ophthalmol. 2018 Apr 18;11(4):559-566. doi: 10.18240/ijo.2018.04.04. eCollection 2018. Int J Ophthalmol. 2018. PMID: 29675371 Free PMC article.
-
Recent advances in engineered exosome-based therapies for ocular vascular disease.J Nanobiotechnology. 2025 Jul 19;23(1):526. doi: 10.1186/s12951-025-03589-3. J Nanobiotechnology. 2025. PMID: 40684186 Free PMC article. Review.
References
-
- Sayegh RG,, Kiss CG,, Simader C,, et al. A systematic correlation of morphology and function using spectral domain optical coherence tomography and microperimetry in patients with geographic atrophy. Br J Ophthalmol. 2014; 98: 1050–1055. - PubMed
-
- Sunness JS,, Gonzalez-Baron J,, Applegate CA,, et al. Enlargement of atrophy and visual acuity loss in the geographic atrophy form of age-related macular degeneration. Ophthalmology. 1999; 106: 1768–1779. - PubMed
-
- Schatz H,, McDonald HR. Atrophic macular degeneration. Rate of spread of geographic atrophy and visual loss. Ophthalmology. 1989; 96: 1541–1551. - PubMed
-
- Kaestel CG,, Lovato P,, Odum N,, Nissen MH,, Ropke C. The immune privilege of the eye: human retinal pigment epithelial cells selectively modulate T-cell activation in vitro. Curr Eye Res. 2005; 30: 375–383. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

