Inactivation of Cytomegalovirus in Breast Milk Using Ultraviolet-C Irradiation: Opportunities for a New Treatment Option in Breast Milk Banking
- PMID: 27537346
- PMCID: PMC4990219
- DOI: 10.1371/journal.pone.0161116
Inactivation of Cytomegalovirus in Breast Milk Using Ultraviolet-C Irradiation: Opportunities for a New Treatment Option in Breast Milk Banking
Abstract
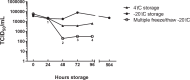
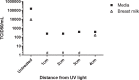
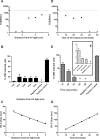
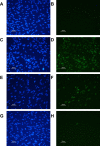
Pasteurized donor human milk is provided by milk banks to very preterm babies where their maternal supply is insufficient or unavailable. Donor milk is currently processed by Holder pasteurization, producing a microbiologically safe product but significantly reducing immunoprotective components. Ultraviolet-C (UV-C) irradiation at 254 nm is being investigated as an alternative treatment method and has been shown to preserve components such as lactoferrin, lysozyme and secretory IgA considerably better than Holder pasteurization. We describe the inactivation of cytomegalovirus, a virus commonly excreted into breast milk, using UV-C irradiation. Full replication was ablated by various treatment doses. However, evidence of viral immediate early proteins within the cells was never completely eliminated indicating that some viral gene transcription was still occurring. In conclusion, UV-C may be a safe alternative to pasteurisation for the treatment of human donor milk that preserves the bioactivity. However, our data suggests that CMV inactivation will have to be carefully evaluated for each device designed to treat breast milk using UV-C irradiation.
Conflict of interest statement
Figures
Similar articles
-
The effect of UV-C pasteurization on bacteriostatic properties and immunological proteins of donor human milk.PLoS One. 2013 Dec 23;8(12):e85867. doi: 10.1371/journal.pone.0085867. eCollection 2013. PLoS One. 2013. PMID: 24376898 Free PMC article.
-
Thermoultrasonication, ultraviolet-C irradiation, and high-pressure processing: Novel techniques to preserve insulin in donor human milk.Clin Nutr. 2021 Nov;40(11):5655-5658. doi: 10.1016/j.clnu.2021.09.028. Epub 2021 Sep 23. Clin Nutr. 2021. PMID: 34666256
-
High Hydrostatic Pressure Processing Better Preserves the Nutrient and Bioactive Compound Composition of Human Donor Milk.J Nutr. 2019 Mar 1;149(3):497-504. doi: 10.1093/jn/nxy302. J Nutr. 2019. PMID: 30770541 Free PMC article.
-
Innovative Techniques of Processing Human Milk to Preserve Key Components.Nutrients. 2019 May 24;11(5):1169. doi: 10.3390/nu11051169. Nutrients. 2019. PMID: 31137691 Free PMC article. Review.
-
Human Milk-Treatment and Quality of Banked Human Milk.Clin Perinatol. 2017 Mar;44(1):95-119. doi: 10.1016/j.clp.2016.11.003. Epub 2016 Dec 27. Clin Perinatol. 2017. PMID: 28159212 Review.
Cited by
-
Viruses and Human Milk: Transmission or Protection?Adv Nutr. 2023 Nov;14(6):1389-1415. doi: 10.1016/j.advnut.2023.08.007. Epub 2023 Aug 20. Adv Nutr. 2023. PMID: 37604306 Free PMC article. Review.
-
Impact of vaccination during pregnancy and staphylococci concentration on the presence of Bacillus cereus in raw human milk.J Perinatol. 2020 Sep;40(9):1323-1330. doi: 10.1038/s41372-019-0586-4. Epub 2020 Jan 9. J Perinatol. 2020. PMID: 31919400 Free PMC article.
-
Observational study of cytomegalovirus from breast milk and necrotising enterocolitis.Arch Dis Child Fetal Neonatal Ed. 2019 Jul 20;105(3):259-65. doi: 10.1136/archdischild-2018-316613. Online ahead of print. Arch Dis Child Fetal Neonatal Ed. 2019. PMID: 31326920 Free PMC article.
-
High prevalence of breastmilk-acquired cytomegalovirus infection in jaundiced infants.J Clin Lab Anal. 2020 Feb;34(2):e23199. doi: 10.1002/jcla.23199. Epub 2020 Jan 29. J Clin Lab Anal. 2020. PMID: 31997475 Free PMC article.
-
Preservation of Anti-cytomegalovirus Activity in Human Milk Following High-Pressure Processing Compared to Holder Pasteurization.Front Nutr. 2022 May 19;9:918814. doi: 10.3389/fnut.2022.918814. eCollection 2022. Front Nutr. 2022. PMID: 35662924 Free PMC article.
References
-
- Hartmann BT, Pang WW, Keil AD, Hartmann PE, Simmer K (2007) Best practice guidelines for the operation of a donor human milk bank in an Australian NICU. Early Human Development 83: 667–673. - PubMed
-
- Franklin JG (1965) A comparison of the bactericidal efficiencies of laboratory Holder and HTST methods of milk pasteurization and the keeping qualities of the processed milks. International Journal of Dairy Technology 18: 115–118.
-
- Hamprecht K, Maschmann J, Muller D, Dietz K, Besenthal I, Goelz R, et al. (2004) Cytomegalovirus (CMV) inactivation in breast milk: Reassessment of pasteurization and freeze-thawing. Pediatric Research 56: 529–535. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

