Experience-Dependent Plasticity Drives Individual Differences in Pheromone-Sensing Neurons
- PMID: 27537487
- PMCID: PMC5003430
- DOI: 10.1016/j.neuron.2016.07.034
Experience-Dependent Plasticity Drives Individual Differences in Pheromone-Sensing Neurons
Abstract
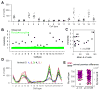
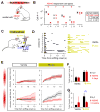
Different individuals exhibit distinct behaviors, but studying the neuronal basis of individuality is a daunting challenge. Here, we considered this question in the vomeronasal organ, a pheromone-detecting epithelium containing hundreds of distinct neuronal types. Using light-sheet microscopy, we characterized in each animal the abundance of 17 physiologically defined types, altogether recording from half a million sensory neurons. Inter-animal differences were much larger than predicted by chance, and different physiological cell types showed distinct patterns of variability. One neuronal type was present in males and nearly absent in females. Surprisingly, this apparent sexual dimorphism was generated by plasticity, as exposure to female scents or single ligands led to both the elimination of this cell type and alterations in olfactory behavior. That an all-or-none apparent sex difference in neuronal types is controlled by experience-even in a sensory system devoted to "innate" behaviors-highlights the extraordinary role of "nurture" in neural individuality.
Copyright © 2016 Elsevier Inc. All rights reserved.
Figures





Similar articles
-
Pheromone sensing in mice.Results Probl Cell Differ. 2009;47:77-96. doi: 10.1007/400_2008_8. Results Probl Cell Differ. 2009. PMID: 19083125 Review.
-
Diverse systems for pheromone perception: multiple receptor families in two olfactory systems.Zoolog Sci. 2008 Dec;25(12):1179-89. doi: 10.2108/zsj.25.1179. Zoolog Sci. 2008. PMID: 19267644 Review.
-
State-dependent responses to sex pheromones in mouse.Curr Opin Neurobiol. 2016 Jun;38:74-9. doi: 10.1016/j.conb.2016.04.001. Epub 2016 Apr 16. Curr Opin Neurobiol. 2016. PMID: 27093585 Free PMC article. Review.
-
Coding of pheromones by vomeronasal receptors.Cell Tissue Res. 2021 Jan;383(1):367-386. doi: 10.1007/s00441-020-03376-6. Epub 2021 Jan 12. Cell Tissue Res. 2021. PMID: 33433690 Review.
-
Encoding pheromonal signals in the accessory olfactory bulb of behaving mice.Science. 2003 Feb 21;299(5610):1196-201. doi: 10.1126/science.1082133. Science. 2003. PMID: 12595684
Cited by
-
Sexually dimorphic architecture and function of a mechanosensory circuit in C. elegans.Nat Commun. 2022 Nov 11;13(1):6825. doi: 10.1038/s41467-022-34661-3. Nat Commun. 2022. PMID: 36369281 Free PMC article.
-
Gene expression profiling of the olfactory tissues of sex-separated and sex-combined female and male mice.Sci Data. 2018 Dec 4;5:180260. doi: 10.1038/sdata.2018.260. Sci Data. 2018. PMID: 30512012 Free PMC article.
-
The Bruce effect: Representational stability and memory formation in the accessory olfactory bulb of the female mouse.Cell Rep. 2022 Aug 23;40(8):111262. doi: 10.1016/j.celrep.2022.111262. Cell Rep. 2022. PMID: 36001975 Free PMC article.
-
Fighting Females: Neural and Behavioral Consequences of Social Defeat Stress in Female Mice.Biol Psychiatry. 2019 Nov 1;86(9):657-668. doi: 10.1016/j.biopsych.2019.05.005. Epub 2019 May 13. Biol Psychiatry. 2019. PMID: 31255250 Free PMC article.
-
Sex steroid hormone synthesis, metabolism, and the effects on the mammalian olfactory system.Cell Tissue Res. 2023 Jan;391(1):19-42. doi: 10.1007/s00441-022-03707-9. Epub 2022 Nov 19. Cell Tissue Res. 2023. PMID: 36401093 Free PMC article. Review.
References
-
- Alekseyenko OV, Baum MJ, Cherry JA. Sex and gonadal steroid modulation of pheromone receptor gene expression in the mouse vomeronasal organ. Neuroscience. 2006;140:1349–57. - PubMed
-
- Axelson M, Graham CE, Sjövall J. Identification and quantitation of steroids in sulfate fractions from plasma of pregnant chimpanzee, orangutan, and rhesus monkey. Endocrinology. 1984;114:337–44. - PubMed
-
- Bean NJ, Wysocki CJ. Vomeronasal organ removal and female mouse aggression: the role of experience. Physiol Behav. 1989;45:875–82. - PubMed
-
- Broad KD, Keverne EB. The post-natal chemosensory environment induces epigenetic changes in vomeronasal receptor gene expression and a bias in olfactory preference. Behav Genet. 2012;42:461–71. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

