T315 Decreases Acute Myeloid Leukemia Cell Viability through a Combination of Apoptosis Induction and Autophagic Cell Death
- PMID: 27537872
- PMCID: PMC5000734
- DOI: 10.3390/ijms17081337
T315 Decreases Acute Myeloid Leukemia Cell Viability through a Combination of Apoptosis Induction and Autophagic Cell Death
Abstract
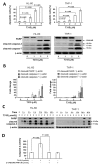
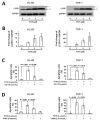
T315, an integrin-linked kinase (ILK) inhibitor, has been shown to suppress the proliferation of breast cancer, stomach cancer and chronic lymphocytic leukemia cells. Here we demonstrate that T315 decreases cell viability of acute myeloid leukemia (AML) cell lines (HL-60 and THP-1) and primary leukemia cells from AML patients in a dose-responsive manner. Normal human bone marrow cells are less sensitive than leukemia cells to T315. T315 down regulates protein kinase B (Akt) and p-Akt and induces caspase activation, poly-ADP-ribose polymerase (PARP) cleavage, apoptosis and autophagy through an ILK-independent manner. Interestingly, pretreatment with autophagy inhibitors rescues cells from apoptosis and concomitant PARP cleavage, which implicates a key role of autophagic cell death in T315-mediated cytotoxicity. T315 also demonstrates efficacy in vivo, suppressing the growth of THP-1 xenograft tumors in athymic nude mice when administered intraperitoneally. This study shows that autophagic cell death and apoptosis cooperatively contribute to the anticancer activity of T315 in AML cells. In conclusion, the complementary roles of apoptotic and autophagic cell death should be considered in the future assessment of the translational value of T315 in AML therapy.
Keywords: T315; acute myeloid leukemia; apoptosis; autophagic cell death; autophagy.
Figures
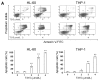
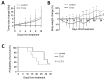
 ) or 48 h (□). The cells were analyzed by MTS assay, as described in Materials and Methods; (C) Primary AML cells (0.25 × 106 cells/mL) were incubated with T315 or DMSO for 24 h. The cells were stained with annexin V-FITC and PI to assess apoptotic cells percentage (n = 26); (D) Normal bone marrow nucleated cells (0.25 × 106 cells/mL) were incubated with T315 or DMSO for 24 h. The cells were stained with annexin V-FITC and PI to assess apoptotic cells percentage (n = 16). * denotes p < 0.05; ** denotes p < 0.01 compared to the control group (in panel A) or compared to the primary AML cells at the same concentration of T315 (in panel D).
) or 48 h (□). The cells were analyzed by MTS assay, as described in Materials and Methods; (C) Primary AML cells (0.25 × 106 cells/mL) were incubated with T315 or DMSO for 24 h. The cells were stained with annexin V-FITC and PI to assess apoptotic cells percentage (n = 26); (D) Normal bone marrow nucleated cells (0.25 × 106 cells/mL) were incubated with T315 or DMSO for 24 h. The cells were stained with annexin V-FITC and PI to assess apoptotic cells percentage (n = 16). * denotes p < 0.05; ** denotes p < 0.01 compared to the control group (in panel A) or compared to the primary AML cells at the same concentration of T315 (in panel D).
 ) or 48 h (□). The cells were analyzed by MTS assay, as described in Materials and Methods; (C) Primary AML cells (0.25 × 106 cells/mL) were incubated with T315 or DMSO for 24 h. The cells were stained with annexin V-FITC and PI to assess apoptotic cells percentage (n = 26); (D) Normal bone marrow nucleated cells (0.25 × 106 cells/mL) were incubated with T315 or DMSO for 24 h. The cells were stained with annexin V-FITC and PI to assess apoptotic cells percentage (n = 16). * denotes p < 0.05; ** denotes p < 0.01 compared to the control group (in panel A) or compared to the primary AML cells at the same concentration of T315 (in panel D).
) or 48 h (□). The cells were analyzed by MTS assay, as described in Materials and Methods; (C) Primary AML cells (0.25 × 106 cells/mL) were incubated with T315 or DMSO for 24 h. The cells were stained with annexin V-FITC and PI to assess apoptotic cells percentage (n = 26); (D) Normal bone marrow nucleated cells (0.25 × 106 cells/mL) were incubated with T315 or DMSO for 24 h. The cells were stained with annexin V-FITC and PI to assess apoptotic cells percentage (n = 16). * denotes p < 0.05; ** denotes p < 0.01 compared to the control group (in panel A) or compared to the primary AML cells at the same concentration of T315 (in panel D).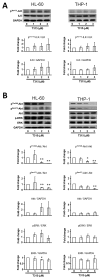


Similar articles
-
OSU-A9, an indole-3-carbinol derivative, induces cytotoxicity in acute myeloid leukemia through reactive oxygen species-mediated apoptosis.Biochem Pharmacol. 2013 Nov 15;86(10):1430-40. doi: 10.1016/j.bcp.2013.09.002. Epub 2013 Sep 13. Biochem Pharmacol. 2013. PMID: 24041743
-
Targeting PDK1 with dichloroacetophenone to inhibit acute myeloid leukemia (AML) cell growth.Oncotarget. 2016 Jan 12;7(2):1395-407. doi: 10.18632/oncotarget.6366. Oncotarget. 2016. PMID: 26593251 Free PMC article.
-
Quercetin simultaneously induces G0 /G1 -phase arrest and caspase-mediated crosstalk between apoptosis and autophagy in human leukemia HL-60 cells.Environ Toxicol. 2017 Jul;32(7):1857-1868. doi: 10.1002/tox.22408. Epub 2017 Mar 2. Environ Toxicol. 2017. PMID: 28251795
-
Mechanisms and consequences of constitutive activation of integrin-linked kinase in acute myeloid leukemia.Cytokine Growth Factor Rev. 2018 Oct;43:1-7. doi: 10.1016/j.cytogfr.2018.06.001. Epub 2018 Jun 7. Cytokine Growth Factor Rev. 2018. PMID: 29903521 Review.
-
Role of autophagy in acute myeloid leukemia therapy.Chin J Cancer. 2013 Mar;32(3):130-5. doi: 10.5732/cjc.012.10073. Epub 2012 Aug 2. Chin J Cancer. 2013. PMID: 22854065 Free PMC article. Review.
Cited by
-
Tanshinone IIA regulates human AML cell proliferation, cell cycle, and apoptosis through miR-497-5p/AKT3 axis.Cancer Cell Int. 2020 Aug 7;20:379. doi: 10.1186/s12935-020-01468-5. eCollection 2020. Cancer Cell Int. 2020. PMID: 32782437 Free PMC article.
-
Protein Kinase Inhibitors as Therapeutic Drugs in AML: Advances and Challenges.Curr Pharm Des. 2017 Nov 16;23(29):4303-4310. doi: 10.2174/1381612823666170703164114. Curr Pharm Des. 2017. PMID: 28671056 Free PMC article. Review.
-
TCP1 increases drug resistance in acute myeloid leukemia by suppressing autophagy via activating AKT/mTOR signaling.Cell Death Dis. 2021 Nov 8;12(11):1058. doi: 10.1038/s41419-021-04336-w. Cell Death Dis. 2021. PMID: 34750375 Free PMC article.
-
microRNA-628 inhibits the proliferation of acute myeloid leukemia cells by directly targeting IGF-1R.Onco Targets Ther. 2019 Jan 29;12:907-919. doi: 10.2147/OTT.S192137. eCollection 2019. Onco Targets Ther. 2019. Retraction in: Onco Targets Ther. 2021 Sep 19;14:4847-4848. doi: 10.2147/OTT.S339844. PMID: 30774377 Free PMC article. Retracted.
-
ILK inhibition reduces osteophyte formation through suppression of osteogenesis in BMSCs via Akt/GSK-3β/β-catenin pathway.Mol Biol Rep. 2024 Mar 14;51(1):421. doi: 10.1007/s11033-024-09336-5. Mol Biol Rep. 2024. PMID: 38483756
References
-
- Hsu E.C., Kulp S.K., Huang H.L., Tu H.J., Salunke S.B., Sullivan N.J., Sun D., Wicha M.S., Shapiro C.L., Chen C.S. Function of integrin-linked kinase in modulating the stemness of IL-6-abundant breast cancer cells by regulating γ-secretase-mediated NOTCH1 activation in caveolae. Neoplasia. 2015;17:497–508. doi: 10.1016/j.neo.2015.06.001. - DOI - PMC - PubMed
-
- Tseng P.C., Chen C.L., Shan Y.S., Chang W.T., Liu H.S., Hong T.M., Hsieh C.Y., Lin S.H., Lin C.F. An increase in integrin-linked kinase non-canonically confers NF-κB-mediated growth advantages to gastric cancer cells by activating ERK1/2. Cell Commun. Signal. 2014;12:69. doi: 10.1186/s12964-014-0069-3. - DOI - PMC - PubMed
-
- Liu T.M., Ling Y., Woyach J.A., Beckwith K., Yeh Y.Y., Hertlein E., Zhang X., Lehman A., Awan F., Jones J.A., et al. OSU-T315: A novel targeted therapeutic that antagonizes AKT membrane localization and activation of chronic lymphocytic leukemia cells. Blood. 2015;125:284–295. doi: 10.1182/blood-2014-06-583518. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

