Effects of the pulsed electromagnetic field PST® on human tendon stem cells: a controlled laboratory study
- PMID: 27538432
- PMCID: PMC4989537
- DOI: 10.1186/s12906-016-1261-3
Effects of the pulsed electromagnetic field PST® on human tendon stem cells: a controlled laboratory study
Abstract
Background: Current clinical procedures for rotator cuff tears need to be improved, as a high rate of failure is still observed. Therefore, new approaches have been attempted to stimulate self-regeneration, including biophysical stimulation modalities, such as low-frequency pulsed electromagnetic fields, which are alternative and non-invasive methods that seem to produce satisfying therapeutic effects. While little is known about their mechanism of action, it has been speculated that they may act on resident stem cells. Thus, the purpose of this study was to evaluate the effects of a pulsed electromagnetic field (PST®) on human tendon stem cells (hTSCs) in order to elucidate the possible mechanism of the observed therapeutic effects.
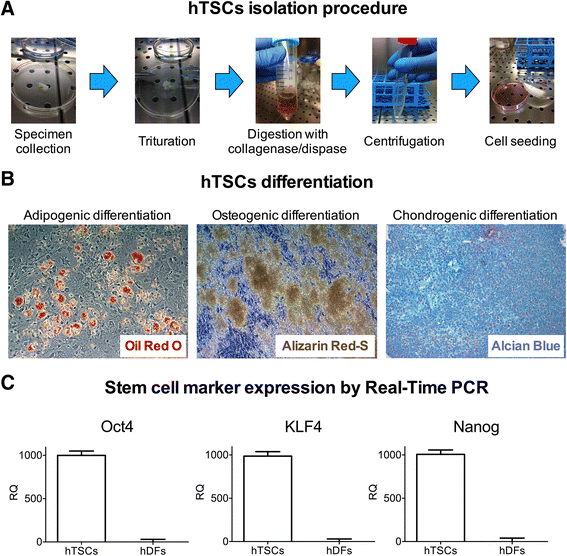
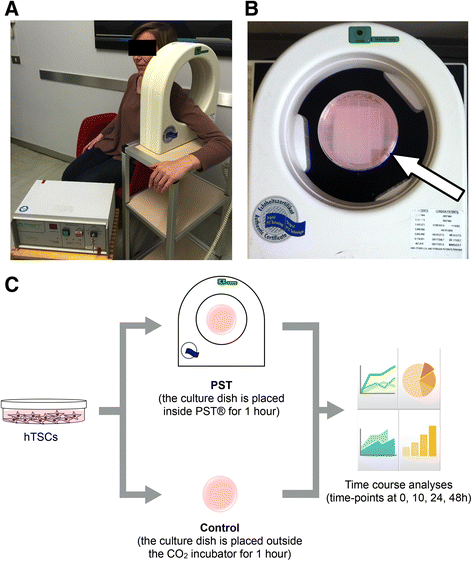
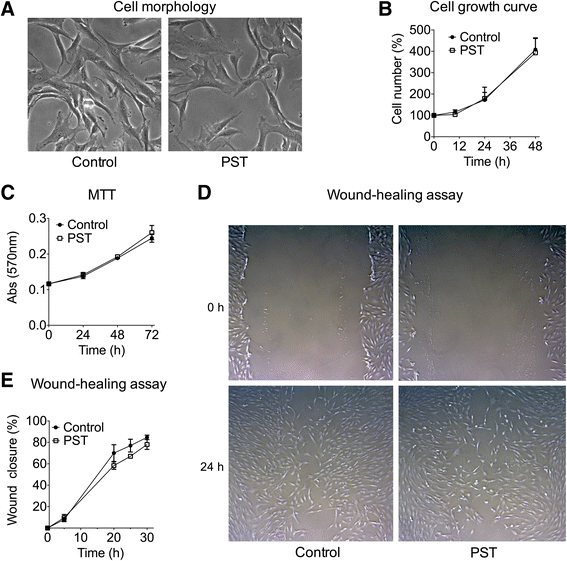
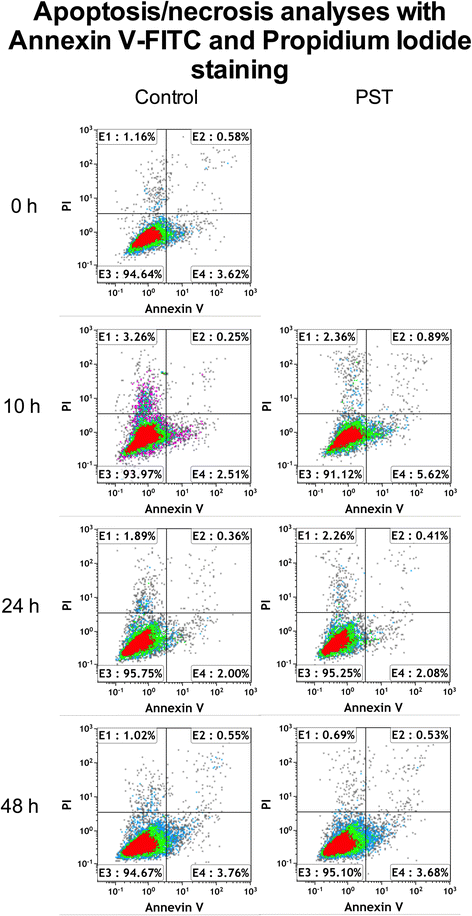
Methods: hTSCs from the rotator cuff were isolated from tendon biopsies and cultured in vitro. Then, cells were exposed to a 1-h PST® treatment and compared to control untreated cells in terms of cell morphology, proliferation, viability, migration, and stem cell marker expression.
Results: Exposure of hTSCs to PST® did not cause any significant changes in proliferation, viability, migration, and morphology. Instead, while stem cell marker expression significantly decreased in control cells during cell culturing, PST®-treated cells did not have a significant reduction of the same markers.
Conclusions: While PST® did not have significant effects on hTSCs proliferation, the treatment had beneficial effects on stem cell marker expression, as treated cells maintained a higher expression of these markers during culturing. These results support the notion that PST® treatment may increase the patient stem cell regenerative potential.
Keywords: Pulsed electromagnetic fields; Pulsed signal therapy; Rotator cuff; Tendon stem cells.
Figures

Similar articles
-
Isolation and characterization of 2 new human rotator cuff and long head of biceps tendon cells possessing stem cell-like self-renewal and multipotential differentiation capacity.Am J Sports Med. 2013 Jul;41(7):1653-64. doi: 10.1177/0363546512473572. Epub 2013 Feb 7. Am J Sports Med. 2013. PMID: 23393078
-
Effect of Hypoxia on Self-Renewal Capacity and Differentiation in Human Tendon-Derived Stem Cells.Med Sci Monit. 2017 Mar 17;23:1334-1339. doi: 10.12659/msm.903892. Med Sci Monit. 2017. PMID: 28302994 Free PMC article.
-
Human tendon stem cells better maintain their stemness in hypoxic culture conditions.PLoS One. 2013 Apr 16;8(4):e61424. doi: 10.1371/journal.pone.0061424. Print 2013. PLoS One. 2013. PMID: 23613849 Free PMC article.
-
Cell- and gene-based approaches to tendon regeneration.J Shoulder Elbow Surg. 2012 Feb;21(2):278-94. doi: 10.1016/j.jse.2011.11.015. J Shoulder Elbow Surg. 2012. PMID: 22244071 Review.
-
Review article: Regenerative techniques for repair of rotator cuff tears.J Orthop Surg (Hong Kong). 2013 Aug;21(2):226-31. doi: 10.1177/230949901302100223. J Orthop Surg (Hong Kong). 2013. PMID: 24014790 Review.
Cited by
-
Uncovering the effect of low-frequency static magnetic field on tendon-derived cells: from mechanosensing to tenogenesis.Sci Rep. 2017 Sep 8;7(1):10948. doi: 10.1038/s41598-017-11253-6. Sci Rep. 2017. PMID: 28887547 Free PMC article.
-
Fatty Infiltration Is a Prognostic Marker of Muscle Function After Rotator Cuff Tear.Am J Sports Med. 2018 Jul;46(9):2161-2169. doi: 10.1177/0363546518769267. Epub 2018 May 11. Am J Sports Med. 2018. PMID: 29750541 Free PMC article.
-
Tendinopathy: Pathophysiology, Therapeutic Options, and Role of Nutraceutics. A Narrative Literature Review.Medicina (Kaunas). 2019 Aug 7;55(8):447. doi: 10.3390/medicina55080447. Medicina (Kaunas). 2019. PMID: 31394838 Free PMC article. Review.
-
Role of tendon-derived stem cells in tendon and ligament repair: focus on tissue engineer.Front Bioeng Biotechnol. 2024 Aug 8;12:1357696. doi: 10.3389/fbioe.2024.1357696. eCollection 2024. Front Bioeng Biotechnol. 2024. PMID: 39175617 Free PMC article. Review.
-
A2A adenosine receptors are involved in the reparative response of tendon cells to pulsed electromagnetic fields.PLoS One. 2020 Sep 30;15(9):e0239807. doi: 10.1371/journal.pone.0239807. eCollection 2020. PLoS One. 2020. PMID: 32998161 Free PMC article.
References
-
- Meislin RJ, Sperling JW, Stitik TP. Persistent shoulder pain: epidemiology, pathophysiology, and diagnosis. Am J Orthop (Belle Mead NJ) 2005;34(12 Suppl):5–9. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

