Probing Oxide-Ion Mobility in the Mixed Ionic-Electronic Conductor La2NiO4+δ by Solid-State (17)O MAS NMR Spectroscopy
- PMID: 27538437
- PMCID: PMC5025823
- DOI: 10.1021/jacs.6b07348
Probing Oxide-Ion Mobility in the Mixed Ionic-Electronic Conductor La2NiO4+δ by Solid-State (17)O MAS NMR Spectroscopy
Abstract
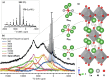
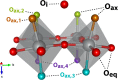
While solid-state NMR spectroscopic techniques have helped clarify the local structure and dynamics of ionic conductors, similar studies of mixed ionic-electronic conductors (MIECs) have been hampered by the paramagnetic behavior of these systems. Here we report high-resolution (17)O (I = 5/2) solid-state NMR spectra of the mixed-conducting solid oxide fuel cell (SOFC) cathode material La2NiO4+δ, a paramagnetic transition-metal oxide. Three distinct oxygen environments (equatorial, axial, and interstitial) can be assigned on the basis of hyperfine (Fermi contact) shifts and quadrupolar nutation behavior, aided by results from periodic DFT calculations. Distinct structural distortions among the axial sites, arising from the nonstoichiometric incorporation of interstitial oxygen, can be resolved by advanced magic angle turning and phase-adjusted sideband separation (MATPASS) NMR experiments. Finally, variable-temperature spectra reveal the onset of rapid interstitial oxide motion and exchange with axial sites at ∼130 °C, associated with the reported orthorhombic-to-tetragonal phase transition of La2NiO4+δ. From the variable-temperature spectra, we develop a model of oxide-ion dynamics on the spectral time scale that accounts for motional differences of all distinct oxygen sites. Though we treat La2NiO4+δ as a model system for a combined paramagnetic (17)O NMR and DFT methodology, the approach presented herein should prove applicable to MIECs and other functionally important paramagnetic oxides.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Enhanced efficiency of solid-state NMR investigations of energy materials using an external automatic tuning/matching (eATM) robot.J Magn Reson. 2017 Feb;275:127-136. doi: 10.1016/j.jmr.2016.12.008. Epub 2016 Dec 23. J Magn Reson. 2017. PMID: 28064071
-
A systematic study of 25Mg NMR in paramagnetic transition metal oxides: applications to Mg-ion battery materials.Phys Chem Chem Phys. 2016 Dec 21;19(1):613-625. doi: 10.1039/c6cp06338a. Phys Chem Chem Phys. 2016. PMID: 27918022
-
Linking local environments and hyperfine shifts: a combined experimental and theoretical (31)P and (7)Li solid-state NMR study of paramagnetic Fe(III) phosphates.J Am Chem Soc. 2010 Dec 1;132(47):16825-40. doi: 10.1021/ja102678r. Epub 2010 Nov 5. J Am Chem Soc. 2010. PMID: 21053901
-
17O NMR Spectroscopy in Lithium-Ion Battery Cathode Materials: Challenges and Interpretation.J Am Chem Soc. 2022 Oct 19;144(41):18714-18729. doi: 10.1021/jacs.2c02927. Epub 2022 Oct 6. J Am Chem Soc. 2022. PMID: 36201656 Free PMC article. Review.
-
Magic angle spinning NMR of paramagnetic proteins.Acc Chem Res. 2013 Sep 17;46(9):2108-16. doi: 10.1021/ar300349y. Epub 2013 Mar 18. Acc Chem Res. 2013. PMID: 23506094 Review.
Cited by
-
Distinguishing faceted oxide nanocrystals with 17O solid-state NMR spectroscopy.Nat Commun. 2017 Sep 18;8(1):581. doi: 10.1038/s41467-017-00603-7. Nat Commun. 2017. PMID: 28924155 Free PMC article.
-
NMR crystallography: structure and properties of materials from solid-state nuclear magnetic resonance observables.IUCrJ. 2017 May 2;4(Pt 4):350-359. doi: 10.1107/S2052252517006042. eCollection 2017 Jul 1. IUCrJ. 2017. PMID: 28875022 Free PMC article. Review.
-
Interplay between A-site and oxygen-vacancy ordering, and mixed electron/oxide-ion conductivity in n = 1 Ruddlesden-Popper perovskite Sr2Nd2Zn2O7.Chem Sci. 2024 Dec 18;16(4):1932-1947. doi: 10.1039/d4sc05323k. eCollection 2025 Jan 22. Chem Sci. 2024. PMID: 39722789 Free PMC article.
-
Revealing the Structure and Oxygen Transport at Interfaces in Complex Oxide Heterostructures via 17O NMR Spectroscopy.Chem Mater. 2020 Sep 22;32(18):7921-7931. doi: 10.1021/acs.chemmater.0c02698. Epub 2020 Aug 19. Chem Mater. 2020. PMID: 32982045 Free PMC article.
-
Disorder and Oxide Ion Diffusion Mechanism in La1.54Sr0.46Ga3O7.27 Melilite from Nuclear Magnetic Resonance.J Am Chem Soc. 2023 Oct 11;145(40):21817-21831. doi: 10.1021/jacs.3c04821. Epub 2023 Oct 2. J Am Chem Soc. 2023. PMID: 37782307 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

