Antibacterial effects of Lactobacillus and bacteriocin PLNC8 αβ on the periodontal pathogen Porphyromonas gingivalis
- PMID: 27538539
- PMCID: PMC4990846
- DOI: 10.1186/s12866-016-0810-8
Antibacterial effects of Lactobacillus and bacteriocin PLNC8 αβ on the periodontal pathogen Porphyromonas gingivalis
Abstract
Background: The complications in healthcare systems associated with antibiotic-resistant microorganisms have resulted in an intense search for new effective antimicrobials. Attractive substances from which novel antibiotics may be developed are the bacteriocins. These naturally occurring peptides are generally considered to be safe and efficient at eliminating pathogenic bacteria. Among specific keystone pathogens in periodontitis, Porphyromonas gingivalis is considered to be the most important pathogen in the development and progression of chronic inflammatory disease. The aim of the present study was to investigate the antimicrobial effects of different Lactobacillus species and the two-peptide bacteriocin PLNC8 αβ on P. gingivalis.
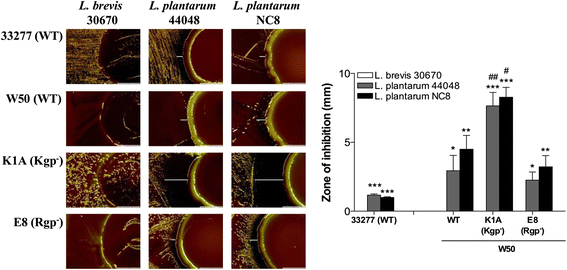
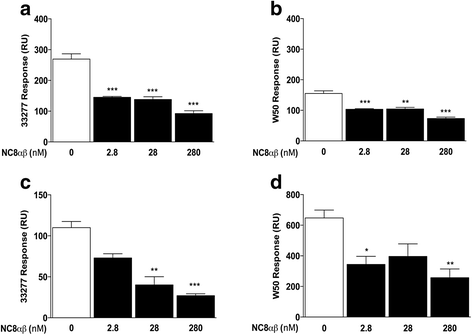
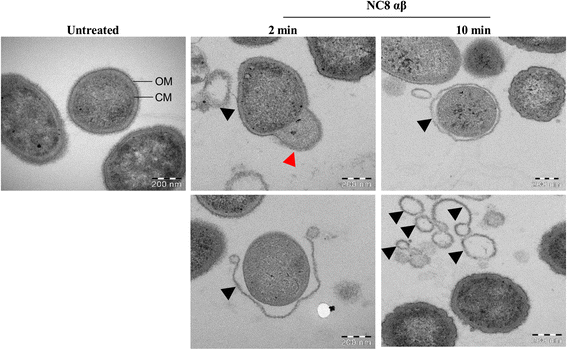
Results: Growth inhibition of P. gingivalis was obtained by viable Lactobacillus and culture media from L. plantarum NC8 and 44048, but not L. brevis 30670. The two-peptide bacteriocin from L. plantarum NC8 (PLNC8 αβ) was found to be efficient against P. gingivalis through binding followed by permeabilization of the membranes, using Surface plasmon resonance analysis and DNA staining with Sytox Green. Liposomal systems were acquired to verify membrane permeabilization by PLNC8 αβ. The antimicrobial activity of PLNC8 αβ was found to be rapid (1 min) and visualized by TEM to cause cellular distortion through detachment of the outer membrane and bacterial lysis.
Conclusion: Soluble or immobilized PLNC8 αβ bacteriocins may be used to prevent P. gingivalis colonization and subsequent pathogenicity, and thus supplement the host immune system against invading pathogens associated with periodontitis.
Keywords: Bacteriocin; Lactobacillus; P. gingivalis; PLNC8; Periodontitis.
Figures




References
-
- Brodala N, Merricks EP, Bellinger DA, Damrongsri D, Offenbacher S, Beck J, Madianos P, Sotres D, Chang YL, Koch G, et al. Porphyromonas gingivalis bacteremia induces coronary and aortic atherosclerosis in normocholesterolemic and hypercholesterolemic pigs. Arterioscler Thromb Vasc Biol. 2005;25(7):1446–1451. doi: 10.1161/01.ATV.0000167525.69400.9c. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

