TLR2 and TLR9 modulate enteric nervous system inflammatory responses to lipopolysaccharide
- PMID: 27538577
- PMCID: PMC4990868
- DOI: 10.1186/s12974-016-0653-0
TLR2 and TLR9 modulate enteric nervous system inflammatory responses to lipopolysaccharide
Abstract
Background: Accumulating evidence suggest that the enteric nervous system (ENS) plays important roles in gastrointestinal inflammatory responses, which could be in part mediated by Toll-like receptor (TLR) activation. The aim of this study was to characterise the expression and functionality of TLR2/4/9 in the ENS.
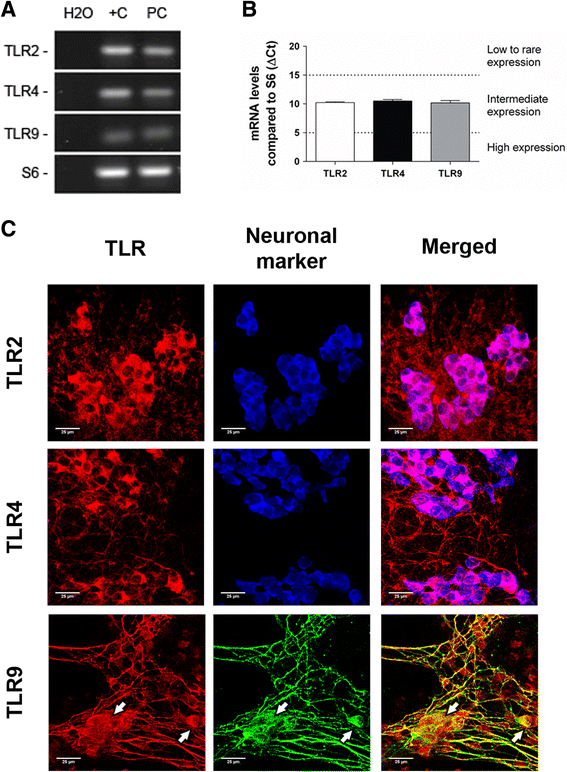
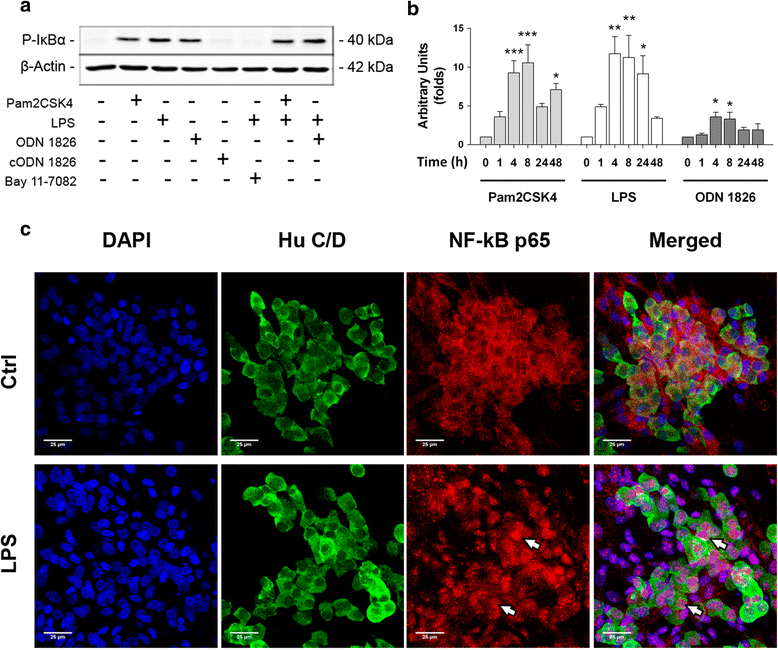
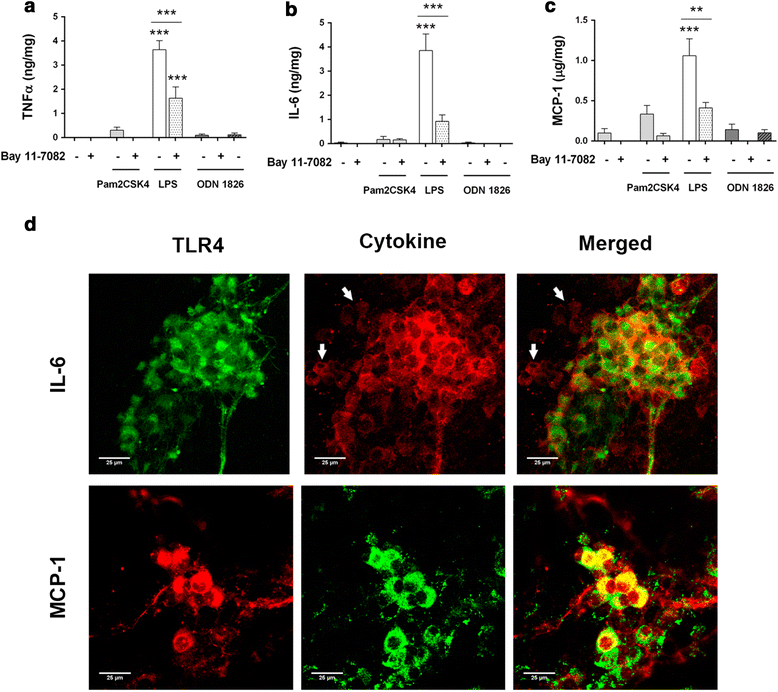
Methods: TLR2/4/9 expression was assessed in the plexuses of adult rats and embryonic ENS cultures by immunofluorescence and quantitative PCR. Following stimulation with TLR2/4/9 ligands or their combinations, activation of NF-kB, production of TNF-α, IL-6 and MCP-1 and chemoattraction of RAW264.7 macrophages were evaluated by means of Western blot, ELISA, immunofluorescence and migration assays in transwell inserts.
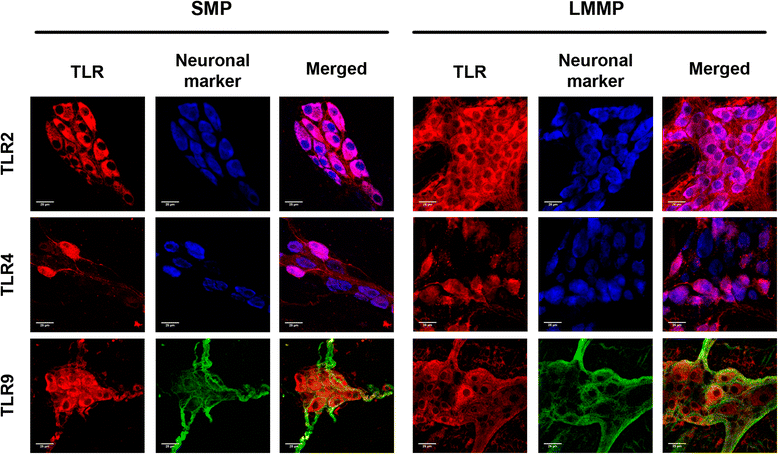
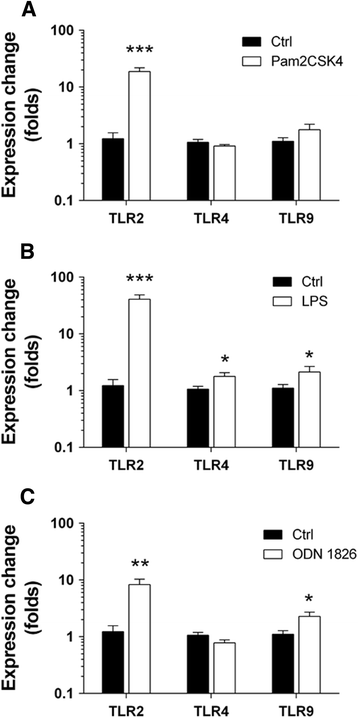
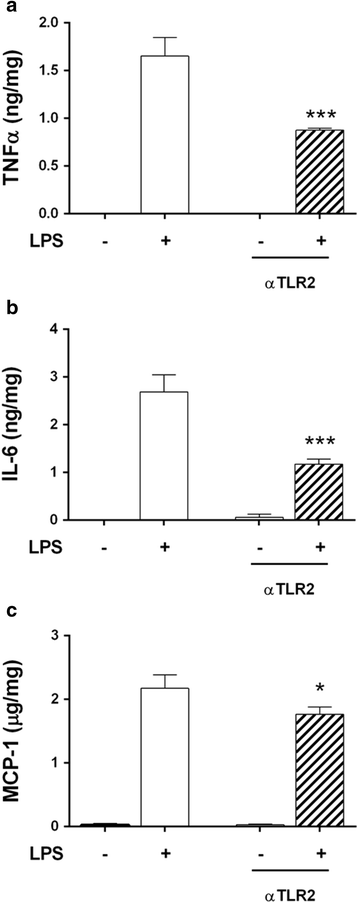
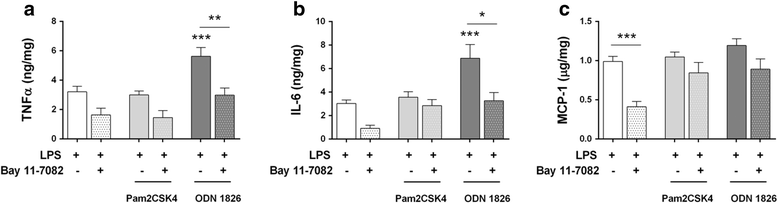
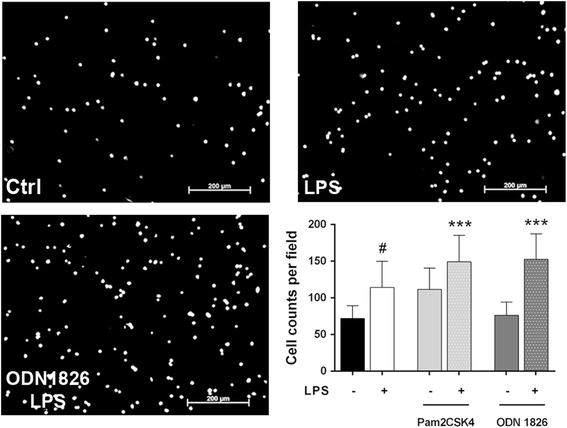
Results: TLR2/4/9 staining colocalised with enteric neuronal markers, whereas their presence in enteroglial processes was low to inexistent. Stimulation of ENS cultures with selective ligands induced NF-kB activation and release of cytokines and chemokines by neurons and resident immunocytes. TLR2 neutralisation before lipopolysaccharide (LPS) challenge reduced production of inflammatory mediators, whereas combination of TLR2/4 ligands promoted macrophage migration. Combined stimulation of cultures with LPS and the CpG oligonucleotide 1826 (TLR4/9 ligands) caused a synergic increase in chemoattraction and cytokine production.
Conclusions: Our results suggest that the ENS, and particularly enteric neurons, can integrate a variety of microbial signals and respond in a relatively selective fashion, depending on the particular TLRs stimulated. These findings additionally suggest that the ENS is capable of initiating a defensive response against pathogens and expanding inflammation.
Keywords: Chemoattraction; Enteric nervous system; Enteric neuron; Inflammation; TLR2; TLR4; TLR9.
Figures
Similar articles
-
Modulation of lipopolysaccharide-induced neuronal response by activation of the enteric nervous system.J Neuroinflammation. 2014 Dec 12;11:202. doi: 10.1186/s12974-014-0202-7. J Neuroinflammation. 2014. PMID: 25497784 Free PMC article.
-
Toll-like receptor 2 regulates intestinal inflammation by controlling integrity of the enteric nervous system.Gastroenterology. 2013 Dec;145(6):1323-33. doi: 10.1053/j.gastro.2013.08.047. Epub 2013 Aug 28. Gastroenterology. 2013. PMID: 23994200
-
Toll like receptor-2 regulates production of glial-derived neurotrophic factors in murine intestinal smooth muscle cells.Mol Cell Neurosci. 2015 Sep;68:24-35. doi: 10.1016/j.mcn.2015.03.018. Epub 2015 Mar 27. Mol Cell Neurosci. 2015. PMID: 25823690
-
Toll-like receptor 2 as a regulator of oral tolerance in the gastrointestinal tract.Mediators Inflamm. 2014;2014:606383. doi: 10.1155/2014/606383. Epub 2014 Sep 17. Mediators Inflamm. 2014. PMID: 25309051 Free PMC article. Review.
-
The gut brain in a dish: Murine primary enteric nervous system cell cultures.Neurogastroenterol Motil. 2022 Feb;34(2):e14215. doi: 10.1111/nmo.14215. Epub 2021 Jul 8. Neurogastroenterol Motil. 2022. PMID: 34236124 Free PMC article. Review.
Cited by
-
Intestinal Bacteria Maintain Adult Enteric Nervous System and Nitrergic Neurons via Toll-like Receptor 2-induced Neurogenesis in Mice.Gastroenterology. 2020 Jul;159(1):200-213.e8. doi: 10.1053/j.gastro.2020.03.050. Epub 2020 Mar 29. Gastroenterology. 2020. PMID: 32234538 Free PMC article.
-
Herpes Simplex Virus Type 1 Infects Enteric Neurons and Triggers Gut Dysfunction via Macrophage Recruitment.Front Cell Infect Microbiol. 2018 Mar 15;8:74. doi: 10.3389/fcimb.2018.00074. eCollection 2018. Front Cell Infect Microbiol. 2018. PMID: 29600197 Free PMC article.
-
Intestinal Region-Specific and Layer-Dependent Induction of TNFα in Rats with Streptozotocin-Induced Diabetes and after Insulin Replacement.Cells. 2021 Sep 13;10(9):2410. doi: 10.3390/cells10092410. Cells. 2021. PMID: 34572059 Free PMC article.
-
Influence of bacterial components on the developmental programming of enteric neurons.Physiol Rep. 2020 Nov;8(21):e14611. doi: 10.14814/phy2.14611. Physiol Rep. 2020. PMID: 33185323 Free PMC article.
-
Gut-brain axis: Mechanisms and potential therapeutic strategies for ischemic stroke through immune functions.Front Neurosci. 2023 Jan 27;17:1081347. doi: 10.3389/fnins.2023.1081347. eCollection 2023. Front Neurosci. 2023. PMID: 36777635 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

