ApoE deficiency promotes colon inflammation and enhances inflammatory potential oxidized-LDL and TNF-α in colon epithelial cells
- PMID: 27538678
- PMCID: PMC5052706
- DOI: 10.1042/BSR20160195
ApoE deficiency promotes colon inflammation and enhances inflammatory potential oxidized-LDL and TNF-α in colon epithelial cells
Erratum in
-
Correction: ApoE deficiency promotes colon inflammation and enhances the inflammatory potential of oxidized-LDL and TNF-α in primary colon epithelial cells.Biosci Rep. 2016 Nov 17;36(6):e00408. doi: 10.1042/BSR20160195COR. Print 2016 Dec. Biosci Rep. 2016. PMID: 27856787 Free PMC article. No abstract available.
Abstract
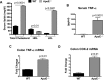
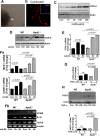
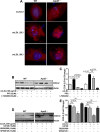
Although deficiency in Apolipoprotein E (ApoE) is linked to many diseases, its effect on colon homeostasis remains unknown. ApoE appears to control inflammation by regulating NF-kB. This study was designed to examine whether ApoE deficiency affects factors of colon integrity in vivo and given the likelihood that ApoE deficiency increases oxidized lipids and TNF-α, this study also examined whether such deficiency enhances the inflammatory potential of oxidized-LDL (oxLDL) and TNF-α, in colon epithelial cells in vitro Here we show that ApoE deficiency is associated with chronic inflammation systemically and in colonic tissues as assessed by TNF-α levels. Increased colon TNF-α mRNA coincided with a substantial increase in cyclooxygenase (COX)-2. ApoE deficiency enhanced the potential of oxLDL and TNF-a to induce COX-2 expression as well as several other inflammatory factors in primary colon epithelial cells. Interestingly, oxLDL enhanced TGF-β expression only in ApoE-/-, but not in wild-type, epithelial cells. ApoE deficiency appears to promote COX-2 expression enhancement through a mechanism that involves persistent NF-κB nuclear localization, PI3 and p38 MAP kinases but independently of Src. In mice, ApoE deficiency promoted a moderate increase in crypt length, which was associated with opposing effects of an increase in cell proliferation and apoptosis at the bottom and top of the crypt, respectively. : Our results support the notion that ApoE plays a central role in colon homeostasis and that ApoE deficiency may constitute a risk factor for colon pathologies.
Keywords: ApoE; colon; inflammation; nuclear factor kappaB; tumour necrosis factors.
©2016 The Author(s).
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

