Early applications of granulocyte colony-stimulating factor (G-CSF) can stabilize the blood-optic-nerve barrier and ameliorate inflammation in a rat model of anterior ischemic optic neuropathy (rAION)
- PMID: 27538969
- PMCID: PMC5087822
- DOI: 10.1242/dmm.025999
Early applications of granulocyte colony-stimulating factor (G-CSF) can stabilize the blood-optic-nerve barrier and ameliorate inflammation in a rat model of anterior ischemic optic neuropathy (rAION)
Abstract
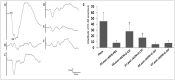
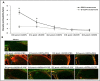
Granulocyte colony-stimulating factor (G-CSF) was reported to have a neuroprotective effect in a rat model of anterior ischemic optic neuropathy (rAION model). However, the therapeutic window and anti-inflammatory effects of G-CSF in a rAION model have yet to be elucidated. Thus, this study aimed to determine the therapeutic window of G-CSF and investigate the mechanisms of G-CSF via regulation of optic nerve (ON) inflammation in a rAION model. Rats were treated with G-CSF on day 0, 1, 2 or 7 post-rAION induction for 5 consecutive days, and a control group were treated with phosphate-buffered saline (PBS). Visual function was assessed by flash visual evoked potentials at 4 weeks post-rAION induction. The survival rate and apoptosis of retinal ganglion cells were determined by FluoroGold labeling and TUNEL assay, respectively. ON inflammation was evaluated by staining of ED1 and Iba1, and ON vascular permeability was determined by Evans Blue extravasation. The type of macrophage polarization was evaluated using quantitative real-time PCR (qRT-PCR). The protein levels of TNF-α and IL-1β were analyzed by western blotting. A therapeutic window during which G-CSF could rescue visual function and retinal ganglion cell survival was demonstrated at day 0 and day 1 post-infarct. Macrophage infiltration was reduced by 3.1- and 1.6-fold by G-CSF treatment starting on day 0 and 1 post-rAION induction, respectively, compared with the PBS-treated group (P<0.05). This was compatible with 3.3- and 1.7-fold reductions in ON vascular permeability after G-CSF treatment compared with PBS treatment (P<0.05). Microglial activation was increased by 3.8- and 3.2-fold in the early (beginning treatment at day 0 or 1) G-CSF-treated group compared with the PBS-treated group (P<0.05). Immediate (within 30 mins of infarct) treatment with G-CSF also induced M2 microglia/macrophage activation. The cytokine levels were lower in the group that received immediate G-CSF treatment compared to those in the later G-CSF treatment group (P<0.05). Early treatment with G-CSF stabilized the blood-ON barrier to reduce macrophage infiltration and induced M2 microglia/macrophage polarization to decrease the expressions of pro-inflammatory cytokines in this rAION model.
Keywords: Blood–optic-nerve barrier; Granulocyte colony-stimulating factor (G-CSF); Macrophage infiltration; Microglia/macrophage polarization; Rat anterior ischemic optic neuropathy (rAION).
© 2016. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures






References
-
- Doycheva D. M., Hadley T., Li L., Applegate R. L. II, Zhang J. H. and Tang J. (2014). Anti-neutrophil antibody enhances the neuroprotective effects of G-CSF by decreasing number of neutrophils in hypoxic ischemic neonatal rat model. Neurobiol. Dis. 69, 192-199. 10.1016/j.nbd.2014.05.024 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

