Bright monomeric near-infrared fluorescent proteins as tags and biosensors for multiscale imaging
- PMID: 27539380
- PMCID: PMC4992171
- DOI: 10.1038/ncomms12405
Bright monomeric near-infrared fluorescent proteins as tags and biosensors for multiscale imaging
Abstract
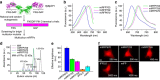
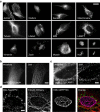
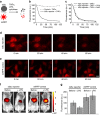
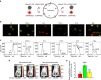
Monomeric near-infrared (NIR) fluorescent proteins (FPs) are in high demand as protein tags and components of biosensors for deep-tissue imaging and multicolour microscopy. We report three bright and spectrally distinct monomeric NIR FPs, termed miRFPs, engineered from bacterial phytochrome, which can be used as easily as GFP-like FPs. miRFPs are 2-5-fold brighter in mammalian cells than other monomeric NIR FPs and perform well in protein fusions, allowing multicolour structured illumination microscopy. miRFPs enable development of several types of NIR biosensors, such as for protein-protein interactions, RNA detection, signalling cascades and cell fate. We demonstrate this by engineering the monomeric fluorescence complementation reporters, the IκBα reporter for NF-κB pathway and the cell cycle biosensor for detection of proliferation status of cells in culture and in animals. miRFPs allow non-invasive visualization and detection of biological processes at different scales, from super-resolution microscopy to in vivo imaging, using the same probes.
Figures

References
-
- Weissleder R. A clearer vision for in vivo imaging. Nat. Biotechnol. 19, 316–317 (2001). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

