SGO1 is involved in the DNA damage response in MYCN-amplified neuroblastoma cells
- PMID: 27539729
- PMCID: PMC4990925
- DOI: 10.1038/srep31615
SGO1 is involved in the DNA damage response in MYCN-amplified neuroblastoma cells
Abstract
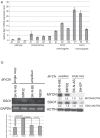
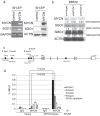
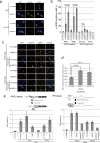
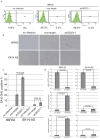
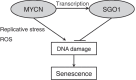
Shugoshin 1 (SGO1) is required for accurate chromosome segregation during mitosis and meiosis; however, its other functions, especially at interphase, are not clearly understood. Here, we found that downregulation of SGO1 caused a synergistic phenotype in cells overexpressing MYCN. Downregulation of SGO1 impaired proliferation and induced DNA damage followed by a senescence-like phenotype only in MYCN-overexpressing neuroblastoma cells. In these cells, SGO1 knockdown induced DNA damage, even during interphase, and this effect was independent of cohesin. Furthermore, MYCN-promoted SGO1 transcription and SGO1 expression tended to be higher in MYCN- or MYC-overexpressing cancers. Together, these findings indicate that SGO1 plays a role in the DNA damage response in interphase. Therefore, we propose that SGO1 represents a potential molecular target for treatment of MYCN-amplified neuroblastoma.
Figures

Similar articles
-
MYCN is amplified during S phase, and c‑myb is involved in controlling MYCN expression and amplification in MYCN‑amplified neuroblastoma cell lines.Mol Med Rep. 2019 Jan;19(1):345-361. doi: 10.3892/mmr.2018.9686. Epub 2018 Nov 22. Mol Med Rep. 2019. PMID: 30483774 Free PMC article.
-
A Novel MYCN-Specific Antigene Oligonucleotide Deregulates Mitochondria and Inhibits Tumor Growth in MYCN-Amplified Neuroblastoma.Cancer Res. 2019 Dec 15;79(24):6166-6177. doi: 10.1158/0008-5472.CAN-19-0008. Epub 2019 Oct 15. Cancer Res. 2019. PMID: 31615807
-
Alternative NHEJ pathway proteins as components of MYCN oncogenic activity in human neural crest stem cell differentiation: implications for neuroblastoma initiation.Cell Death Dis. 2017 Dec 13;8(12):3208. doi: 10.1038/s41419-017-0004-9. Cell Death Dis. 2017. PMID: 29238067 Free PMC article.
-
MYCN oncoprotein targets and their therapeutic potential.Cancer Lett. 2010 Jul 28;293(2):144-57. doi: 10.1016/j.canlet.2010.01.015. Epub 2010 Feb 13. Cancer Lett. 2010. PMID: 20153925 Review.
-
Neuroblastoma-derived v-myc avian myelocytomatosis viral related oncogene or MYCN gene.J Clin Pathol. 2023 Aug;76(8):518-523. doi: 10.1136/jcp-2022-208476. Epub 2023 May 23. J Clin Pathol. 2023. PMID: 37221048 Review.
Cited by
-
SMG6 regulates DNA damage and cell survival in Hippo pathway kinase LATS2-inactivated malignant mesothelioma.Cell Death Discov. 2022 Nov 5;8(1):446. doi: 10.1038/s41420-022-01232-w. Cell Death Discov. 2022. PMID: 36335095 Free PMC article.
-
A Review of the Regulatory Mechanisms of N-Myc on Cell Cycle.Molecules. 2023 Jan 23;28(3):1141. doi: 10.3390/molecules28031141. Molecules. 2023. PMID: 36770809 Free PMC article. Review.
-
Transcriptome Analysis Identifies Novel Mechanisms Associated with the Antitumor Effect of Chitosan-Stabilized Selenium Nanoparticles.Pharmaceutics. 2021 Mar 8;13(3):356. doi: 10.3390/pharmaceutics13030356. Pharmaceutics. 2021. PMID: 33800318 Free PMC article.
-
Overexpression of shugoshin1 predicts a poor prognosis for prostate cancer and promotes metastasis by affecting epithelial-mesenchymal transition.Onco Targets Ther. 2019 Feb 8;12:1111-1118. doi: 10.2147/OTT.S191157. eCollection 2019. Onco Targets Ther. 2019. PMID: 30799941 Free PMC article.
-
Repression of developmental transcription factor networks triggers aging-associated gene expression in human glial progenitor cells.Nat Commun. 2024 May 8;15(1):3873. doi: 10.1038/s41467-024-48118-2. Nat Commun. 2024. PMID: 38719882 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

