The inhibition of EZH2 ameliorates osteoarthritis development through the Wnt/β-catenin pathway
- PMID: 27539752
- PMCID: PMC4990905
- DOI: 10.1038/srep29176
The inhibition of EZH2 ameliorates osteoarthritis development through the Wnt/β-catenin pathway
Abstract
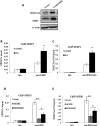
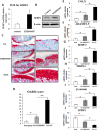
The purpose of our study was to elucidate the role of the histone methyltransferase enhancer of zeste homologue 2 (EZH2) in the pathophysiology of osteoarthritis (OA) and to develop a strategy to modulate EZH2 activity for OA treatment. The expression of EZH2 in normal and OA human cartilage was compared by western blotting. The effect of EZH2 overexpression and inhibition on chondrocyte hypertrophy related gene expression was examined by real-time PCR, and histone methylation on the promoter of the Wnt inhibitor SFRP1 was analyzed using a chromatin immunoprecipitation (ChIP) PCR. Histological assessment of OA mice joint was carried out to assess the in vivo effects of EZH2 inhibitor EPZ005687. We found EZH2 level was significantly increased in the chondrocytes of OA patients compared to normal humans. Overexpression of EZH2 promoted Indian Hedgehog, MMP-13, ADAMTS-5 and COLX expression, while inhibition of EZH2 reversed this trend. Furthermore, the induction of EZH2 led to β-catenin signaling activation by increasing H3K27me3 on the promoter of SFRP1, while the inhibition of EZH2 silenced β-catenin signaling. Finally, intraarticular injection of EPZ005687 delayed OA development in mice. These results implicated EZH2 activity in OA development. Pharmacological inhibition of EZH2 may be an effective therapeutic approach for osteoarthritis.
Figures





Similar articles
-
Verapamil protects against cartilage degradation in osteoarthritis by inhibiting Wnt/β-catenin signaling.PLoS One. 2014 Mar 21;9(3):e92699. doi: 10.1371/journal.pone.0092699. eCollection 2014. PLoS One. 2014. PMID: 24658359 Free PMC article.
-
Targeting β-catenin dependent Wnt signaling via peptidomimetic inhibitors in murine chondrocytes and OA cartilage.Osteoarthritis Cartilage. 2018 Jun;26(6):818-823. doi: 10.1016/j.joca.2018.02.908. Epub 2018 Mar 17. Osteoarthritis Cartilage. 2018. PMID: 29559252
-
EZH2 inhibition reduces cartilage loss and functional impairment related to osteoarthritis.Sci Rep. 2020 Nov 11;10(1):19577. doi: 10.1038/s41598-020-76724-9. Sci Rep. 2020. PMID: 33177650 Free PMC article.
-
Molecular regulation of articular chondrocyte function and its significance in osteoarthritis.Histol Histopathol. 2011 Mar;26(3):377-94. doi: 10.14670/HH-26.377. Histol Histopathol. 2011. PMID: 21210351 Review.
-
The Interaction between microRNAs and the Wnt/β-Catenin Signaling Pathway in Osteoarthritis.Int J Mol Sci. 2021 Sep 13;22(18):9887. doi: 10.3390/ijms22189887. Int J Mol Sci. 2021. PMID: 34576049 Free PMC article. Review.
Cited by
-
Inhibition of EZH2 ameliorates cartilage endplate degeneration and attenuates the progression of intervertebral disc degeneration via demethylation of Sox-9.EBioMedicine. 2019 Oct;48:619-629. doi: 10.1016/j.ebiom.2019.10.006. Epub 2019 Oct 17. EBioMedicine. 2019. PMID: 31631036 Free PMC article.
-
Epigenetic Therapies for Osteoarthritis.Trends Pharmacol Sci. 2020 Aug;41(8):557-569. doi: 10.1016/j.tips.2020.05.008. Epub 2020 Jun 22. Trends Pharmacol Sci. 2020. PMID: 32586653 Free PMC article. Review.
-
MicroRNA-497-5p attenuates IL-1β-induced cartilage matrix degradation in chondrocytes via Wnt/β-catenin signal pathway.Int J Clin Exp Pathol. 2019 Aug 1;12(8):3108-3118. eCollection 2019. Int J Clin Exp Pathol. 2019. PMID: 31934153 Free PMC article.
-
Landscape of Histone Posttranslational Modifications in Osteoarthritis.J Inflamm Res. 2025 Jun 17;18:7893-7906. doi: 10.2147/JIR.S514599. eCollection 2025. J Inflamm Res. 2025. PMID: 40546403 Free PMC article. Review.
-
Loss of histone methyltransferase Ezh2 stimulates an osteogenic transcriptional program in chondrocytes but does not affect cartilage development.J Biol Chem. 2018 Dec 7;293(49):19001-19011. doi: 10.1074/jbc.RA118.003909. Epub 2018 Oct 16. J Biol Chem. 2018. PMID: 30327434 Free PMC article.
References
-
- Lawrence R. C. et al.. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 41, 778–799 (1998). - PubMed
-
- Sassi N. et al.. The roles of canonical and non-canonical Wnt signaling in human de-differentiated articular chondrocytes. Biotech Histochem. 89, 53–65 (2014). - PubMed
-
- Chang C. H. et al.. Anti-inflammatory effects of hydrophilic and lipophilic statins with hyaluronic acid against LPS-induced inflammation in porcine articular chondrocytes. J Orthop Res. 32, 557–565 (2014). - PubMed
-
- Sun M. M. & Beier F. Chondrocyte hypertrophy in skeletal development, growth, and disease. Birth Defects Res C Embryo Today. 102, 74–82 (2014). - PubMed
-
- Goldring M. B. The role of the chondrocyte in osteoarthritis. Arthritis Rheum. 43, 1916–1926 (2000). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

