A Diverse Set of Single-domain Antibodies (VHHs) against the Anthrax Toxin Lethal and Edema Factors Provides a Basis for Construction of a Bispecific Agent That Protects against Anthrax Infection
- PMID: 27539858
- PMCID: PMC5076830
- DOI: 10.1074/jbc.M116.749184
A Diverse Set of Single-domain Antibodies (VHHs) against the Anthrax Toxin Lethal and Edema Factors Provides a Basis for Construction of a Bispecific Agent That Protects against Anthrax Infection
Abstract
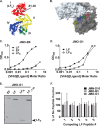
Infection with Bacillus anthracis, the causative agent of anthrax, can lead to persistence of lethal secreted toxins in the bloodstream, even after antibiotic treatment. VHH single-domain antibodies have been demonstrated to neutralize diverse bacterial toxins both in vitro and in vivo, with protein properties such as small size and high stability that make them attractive therapeutic candidates. Recently, we reported on VHHs with in vivo activity against the protective antigen component of the anthrax toxins. Here, we characterized a new set of 15 VHHs against the anthrax toxins that act by binding to the edema factor (EF) and/or lethal factor (LF) components. Six of these VHHs are cross-reactive against both EF and LF and recognize the N-terminal domain (LFN, EFN) of their target(s) with subnanomolar affinity. The cross-reactive VHHs block binding of EF/LF to the protective antigen C-terminal binding interface, preventing toxin entry into the cell. Another VHH appears to recognize the LF C-terminal domain and exhibits a kinetic effect on substrate cleavage by LF. A subset of the VHHs neutralized against EF and/or LF in murine macrophage assays, and the neutralizing VHHs that were tested improved survival of mice in a spore model of anthrax infection. Finally, a bispecific VNA (VHH-based neutralizing agent) consisting of two linked toxin-neutralizing VHHs, JMN-D10 and JMO-G1, was fully protective against lethal anthrax spore infection in mice as a single dose. This set of VHHs should facilitate development of new therapeutic VNAs and/or diagnostic agents for anthrax.
Keywords: EF; LF; VHH; VNA; anthrax toxin; antibody; epitope mapping; microbial pathogenesis; phage display; toxin.
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures





References
-
- Moayeri M., Leppla S. H., Vrentas C., Pomerantsev A. P., and Liu S. (2015) Anthrax pathogenesis. Annu. Rev. Microbiol. 69, 185–208 - PubMed
-
- Moayeri M., Leysath C. E., Tremblay J. M., Vrentas C., Crown D., Leppla S. H., and Shoemaker C. B. (2015) A heterodimer of a VHH (variable domains of camelid heavy chain-only) antibody that inhibits anthrax toxin cell binding linked to a VHH antibody that blocks oligomer formation is highly protective in an anthrax spore challenge model. J. Biol. Chem. 290, 6584–6595 - PMC - PubMed
-
- Muyldermans S. (2013) Nanobodies: natural single-domain antibodies. Annu. Rev. Biochem. 82, 775–797 - PubMed
-
- Hassanzadeh-Ghassabeh G., Devoogdt N., De Pauw P., Vincke C., and Muyldermans S. (2013) Nanobodies and their potential applications. Nanomedicine 8, 1013–1026 - PubMed
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

