Clathrin light chains' role in selective endocytosis influences antibody isotype switching
- PMID: 27540116
- PMCID: PMC5024586
- DOI: 10.1073/pnas.1611189113
Clathrin light chains' role in selective endocytosis influences antibody isotype switching
Abstract
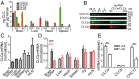
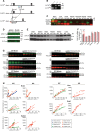
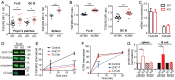
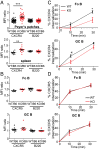
Clathrin, a cytosolic protein composed of heavy and light chain subunits, assembles into a vesicle coat, controlling receptor-mediated endocytosis. To establish clathrin light chain (CLC) function in vivo, we engineered mice lacking CLCa, the major CLC isoform in B lymphocytes, generating animals with CLC-deficient B cells. In CLCa-null mice, the germinal centers have fewer B cells, and they are enriched for IgA-producing cells. This enhanced switch to IgA production in the absence of CLCa was attributable to increased transforming growth factor β receptor 2 (TGFβR2) signaling resulting from defective endocytosis. Internalization of C-X-C chemokine receptor 4 (CXCR4), but not CXCR5, was affected in CLCa-null B cells, and CLC depletion from cell lines affected endocytosis of the δ-opioid receptor, but not the β2-adrenergic receptor, defining a role for CLCs in the uptake of a subset of signaling receptors. This instance of clathrin subunit deletion in vertebrates demonstrates that CLCs contribute to clathrin's role in vivo by influencing cargo selectivity, a function previously assigned exclusively to adaptor molecules.
Keywords: G protein-coupled receptors; TGFβ; antibody isotype switch; clathrin light chain; endocytosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Endocytosis of G protein-coupled receptors is regulated by clathrin light chain phosphorylation.Curr Biol. 2012 Aug 7;22(15):1361-70. doi: 10.1016/j.cub.2012.05.034. Epub 2012 Jun 14. Curr Biol. 2012. PMID: 22704991
-
Differential requirement for type I and type II transforming growth factor beta receptor kinase activity in ligand-mediated receptor endocytosis.J Biol Chem. 1998 Sep 4;273(36):23118-25. doi: 10.1074/jbc.273.36.23118. J Biol Chem. 1998. PMID: 9722540
-
Clathrin light chains are required for the gyrating-clathrin recycling pathway and thereby promote cell migration.Nat Commun. 2014 May 23;5:3891. doi: 10.1038/ncomms4891. Nat Commun. 2014. PMID: 24852344 Free PMC article.
-
The kinase TBK1 controls IgA class switching by negatively regulating noncanonical NF-κB signaling.Nat Immunol. 2012 Nov;13(11):1101-9. doi: 10.1038/ni.2423. Epub 2012 Sep 30. Nat Immunol. 2012. PMID: 23023393 Free PMC article.
-
Genetic analysis of clathrin function in yeast.J Membr Biol. 1990 Jun;116(2):93-105. doi: 10.1007/BF01868668. J Membr Biol. 1990. PMID: 2199679 Review.
Cited by
-
Induced degradation of SNAP-fusion proteins.RSC Chem Biol. 2024 Oct 21;5(12):1232-47. doi: 10.1039/d4cb00184b. Online ahead of print. RSC Chem Biol. 2024. PMID: 39444693 Free PMC article.
-
CLCa mediates a novel cross-talk between Wnt secretion and actin organization.Life Sci Alliance. 2025 May 2;8(7):e202402962. doi: 10.26508/lsa.202402962. Print 2025 Jul. Life Sci Alliance. 2025. PMID: 40316417 Free PMC article.
-
A Clathrin light chain A reporter mouse for in vivo imaging of endocytosis.PLoS One. 2022 Sep 23;17(9):e0273660. doi: 10.1371/journal.pone.0273660. eCollection 2022. PLoS One. 2022. PMID: 36149863 Free PMC article.
-
A conformational switch in clathrin light chain regulates lattice structure and endocytosis at the plasma membrane of mammalian cells.Nat Commun. 2023 Feb 9;14(1):732. doi: 10.1038/s41467-023-36304-7. Nat Commun. 2023. PMID: 36759616 Free PMC article.
-
Clathrin light chains CLCa and CLCb have non-redundant roles in epithelial lumen formation.Life Sci Alliance. 2023 Nov 3;7(1):e202302175. doi: 10.26508/lsa.202302175. Print 2024 Jan. Life Sci Alliance. 2023. PMID: 37923360 Free PMC article.
References
-
- Brodsky FM. Diversity of clathrin function: New tricks for an old protein. Annu Rev Cell Dev Biol. 2012;28:309–336. - PubMed
-
- Huang F, Khvorova A, Marshall W, Sorkin A. Analysis of clathrin-mediated endocytosis of epidermal growth factor receptor by RNA interference. J Biol Chem. 2004;279(16):16657–16661. - PubMed
-
- Ferreira F, et al. Endocytosis of G protein-coupled receptors is regulated by clathrin light chain phosphorylation. Curr Biol. 2012;22(15):1361–1370. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

