Improved skin permeation of methotrexate via nanosized ultradeformable liposomes
- PMID: 27540293
- PMCID: PMC4982511
- DOI: 10.2147/IJN.S109565
Improved skin permeation of methotrexate via nanosized ultradeformable liposomes
Abstract
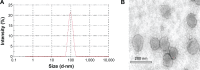
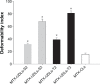
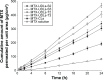
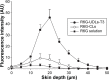
The aim of this study is to investigate methotrexate-entrapped ultradeformable liposomes (MTX-UDLs) for potential transdermal application. MTX-UDLs were prepared by extrusion method with phosphatidylcholine as a bilayer matrix and sodium cholate or Tween 80 as an edge activator. The physicochemical properties of MTX-UDLs were determined in terms of particle size, polydispersity index, zeta potential, and entrapment efficiency. The deformability of MTX-UDLs was compared with that of methotrexate-entrapped conventional liposomes (MTX-CLs) using a steel pressure filter device. The skin permeation of MTX-UDLs was investigated using Franz diffusion cell, and the skin penetration depth of rhodamine 6G-entrapped UDLs was determined by confocal laser scanning microscopy. MTX-UDLs showed a narrow size distribution, with the particle size of ~100 nm. The deformability of MTX-UDLs was two to five times greater than that of MTX-CLs. The skin permeation of MTX-UDLs was significantly improved compared with MTX-CLs and free MTX solution. The optimized UDLs (phosphatidylcholine: Tween 80 =7:3, w/w) showed a higher fluorescence intensity than conventional liposomes at every increment of skin depth. Thus, the optimized UDLs could be promising nanocarriers for systemic delivery of MTX across skin.
Keywords: deformability; methotrexate; skin permeation; transdermal delivery; ultradeformable liposomes.
Figures


Similar articles
-
Enhanced anti-rheumatic activity of methotrexate-entrapped ultradeformable liposomal gel in adjuvant-induced arthritis rat model.Int J Pharm. 2017 Jun 15;525(1):92-100. doi: 10.1016/j.ijpharm.2017.04.032. Epub 2017 Apr 18. Int J Pharm. 2017. PMID: 28428089
-
Perindopril erbumine-entrapped ultradeformable liposomes alleviate sarcopenia via effective skin delivery in muscle atrophy mouse model.Int J Pharm. 2024 Dec 25;667(Pt A):124901. doi: 10.1016/j.ijpharm.2024.124901. Epub 2024 Nov 1. Int J Pharm. 2024. PMID: 39489388
-
Increased skin permeation efficiency of imperatorin via charged ultradeformable lipid vesicles for transdermal delivery.Int J Nanomedicine. 2018 Feb 8;13:831-842. doi: 10.2147/IJN.S150086. eCollection 2018. Int J Nanomedicine. 2018. PMID: 29467573 Free PMC article.
-
Laser-assisted delivery of topical methotrexate - in vitro investigations.Dan Med J. 2016 Jun;63(6):B5254. Dan Med J. 2016. PMID: 27264947 Review.
-
Elastic liposomes as novel carriers: recent advances in drug delivery.Int J Nanomedicine. 2017 Jul 17;12:5087-5108. doi: 10.2147/IJN.S138267. eCollection 2017. Int J Nanomedicine. 2017. PMID: 28761343 Free PMC article. Review.
Cited by
-
The Effect of Alkyl Chain Number in Sucrose Surfactant on the Physical Properties of Quercetin-Loaded Deformable Nanoliposome and Its Effect on In Vitro Human Skin Penetration.Nanomaterials (Basel). 2018 Aug 16;8(8):622. doi: 10.3390/nano8080622. Nanomaterials (Basel). 2018. PMID: 30115875 Free PMC article.
-
Ethosomal gel for rectal transmucosal delivery of domperidone: design of experiment, in vitro, and in vivo evaluation.Drug Deliv. 2022 Dec;29(1):1477-1491. doi: 10.1080/10717544.2022.2072542. Drug Deliv. 2022. PMID: 35543451 Free PMC article.
-
Utilization of PEGylated cerosomes for effective topical delivery of fenticonazole nitrate: in-vitro characterization, statistical optimization, and in-vivo assessment.Drug Deliv. 2021 Dec;28(1):1-9. doi: 10.1080/10717544.2020.1859000. Drug Deliv. 2021. PMID: 33322971 Free PMC article.
-
Plant-Based Nanovesicular Gel Formulations Applied to Skin for Ameliorating the Anti-Inflammatory Efficiency.Gels. 2024 Aug 10;10(8):525. doi: 10.3390/gels10080525. Gels. 2024. PMID: 39195054 Free PMC article. Review.
-
Food-Derived Nanoscopic Drug Delivery Systems for Treatment of Rheumatoid Arthritis.Molecules. 2020 Jul 31;25(15):3506. doi: 10.3390/molecules25153506. Molecules. 2020. PMID: 32752061 Free PMC article. Review.
References
-
- Barry BW. Novel mechanisms and devices to enable successful transdermal drug delivery. Eur J Pharm Sci. 2001;14(2):101–114. - PubMed
-
- Honeywell-Nguyen PL, Bouwstra JA. Vesicles as a tool for transdermal and dermal delivery. Drug Discov Today Technol. 2005;2(1):67–74. - PubMed
-
- Ranade VV, Cannon JB. Drug Delivery Systems. 3rd ed. Boca Raton, FL: CRC Press; 2011.
-
- Barry BW. Dermatological Formulations: Percutaneous Absorption. New York: Marcel Dekker; 1983.
-
- Karande P, Mitragotri S. Enhancement of transdermal drug delivery via synergistic action of chemicals. Biochim Biophys Acta. 2009;1788(11):2362–2373. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

