Reactive oxygen species-mediated neurodegeneration is independent of the ryanodine receptor in Caernorhabditis elegans
- PMID: 27540332
- PMCID: PMC4987101
Reactive oxygen species-mediated neurodegeneration is independent of the ryanodine receptor in Caernorhabditis elegans
Abstract
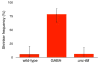
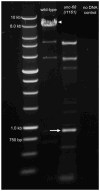
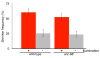
Despite the significant impacts on human health caused by neurodegeneration, our understanding of the degeneration process is incomplete. The nematode Caenorhabditis elegans is emerging as a genetic model organism well suited for identification of conserved cellular mechanisms and molecular pathways of neurodegeneration. Studies in the worm have identified factors that contribute to neurodegeneration, including excitotoxicity and stress due to reactive oxygen species (ROS). Disruption of the gene unc-68, which encodes the ryanodine receptor, abolishes excitotoxic cell death, indicating a role for calcium (Ca2+) signaling in neurodegeneration. We tested the requirement for unc-68 in ROS-mediated neurodegeneration using the genetically encoded photosensitizer KillerRed. Upon illumination of KillerRed expressing animals to produce ROS, we observed similar levels of degeneration in wild-type and unc-68 mutant strains. Our results indicate that ROS-mediated cell death is independent of unc-68 and suggest multiple molecular pathways of neurodegeneration.
Figures


Similar articles
-
Genetic analysis of KillerRed in C. elegans identifies a shared role of calcium genes in ROS-mediated neurodegeneration.J Neurogenet. 2019 Mar;33(1):1-9. doi: 10.1080/01677063.2018.1531857. Epub 2018 Nov 29. J Neurogenet. 2019. PMID: 30489172 Free PMC article.
-
The Role of Ca2+ Signaling in Aging and Neurodegeneration: Insights from Caenorhabditis elegans Models.Cells. 2020 Jan 14;9(1):204. doi: 10.3390/cells9010204. Cells. 2020. PMID: 31947609 Free PMC article. Review.
-
Deleterious effects of mitochondrial ROS generated by KillerRed photodynamic action in human cell lines and C. elegans.J Photochem Photobiol B. 2012 Dec 5;117:1-12. doi: 10.1016/j.jphotobiol.2012.08.005. Epub 2012 Aug 22. J Photochem Photobiol B. 2012. PMID: 23000754
-
Genetic analysis of ryanodine receptor function in Caenorhabditis elegans based on unc-68 revertants.Mol Genet Genomics. 2003 Sep;269(6):797-806. doi: 10.1007/s00438-003-0892-5. Epub 2003 Jul 30. Mol Genet Genomics. 2003. PMID: 12898220
-
Neurodegeneration in excitotoxicity, global cerebral ischemia, and target deprivation: A perspective on the contributions of apoptosis and necrosis.Brain Res Bull. 1998 Jul 1;46(4):281-309. doi: 10.1016/s0361-9230(98)00024-0. Brain Res Bull. 1998. PMID: 9671259 Review.
Cited by
-
Partitioning of naturally-occurring radionuclides (NORM) in Marcellus Shale produced fluids influenced by chemical matrix.Environ Sci Process Impacts. 2016 Apr;18(4):456-63. doi: 10.1039/c5em00540j. Epub 2016 Mar 8. Environ Sci Process Impacts. 2016. PMID: 26952871 Free PMC article.
-
Genetic analysis of KillerRed in C. elegans identifies a shared role of calcium genes in ROS-mediated neurodegeneration.J Neurogenet. 2019 Mar;33(1):1-9. doi: 10.1080/01677063.2018.1531857. Epub 2018 Nov 29. J Neurogenet. 2019. PMID: 30489172 Free PMC article.
-
The Role of Ca2+ Signaling in Aging and Neurodegeneration: Insights from Caenorhabditis elegans Models.Cells. 2020 Jan 14;9(1):204. doi: 10.3390/cells9010204. Cells. 2020. PMID: 31947609 Free PMC article. Review.
References
-
- Azevedo FAC, Carvalho LRB, Grinberg LT, Farfel JM, Ferretti REL, Leite REP, Filho WJ, Lent R, Herculano-Houzel S. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol. 2009;513:532–541. - PubMed
-
- Bulina ME, Chudakov DM, Britanova OV, Yanushevich YG, Staroverov DB, Chepurnykh TV, Merzlyak EM, Shkrob MA, Lukyanov S, Lukyanov KA. A genetically encoded photosensitizer. Nat Biotechnol. 2006;24:95–99. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
