Lowered pH Leads to Fusion Peptide Release and a Highly Dynamic Intermediate of Influenza Hemagglutinin
- PMID: 27541202
- PMCID: PMC5130312
- DOI: 10.1021/acs.jpcb.6b06775
Lowered pH Leads to Fusion Peptide Release and a Highly Dynamic Intermediate of Influenza Hemagglutinin
Abstract
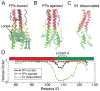
Hemagglutinin (HA), the membrane-bound fusion protein of the influenza virus, enables the entry of virus into host cells via a structural rearrangement. There is strong evidence that the primary trigger for this rearrangement is the low pH environment of a late endosome. To understand the structural basis and the dynamic consequences of the pH trigger, we employed explicit-solvent molecular dynamics simulations to investigate the initial stages of the HA transition. Our results indicate that lowered pH destabilizes HA and speeds up the dissociation of the fusion peptides (FPs). A buried salt bridge between the N-terminus and Asp1122 of HA stem domain locks the FPs and may act as one of the pH sensors. In line with recent observations from simplified protein models, we find that, after the dissociation of FPs, a structural order-disorder transition in a loop connecting the central coiled-coil to the C-terminal domains produces a highly mobile HA. This motion suggests the existence of a long-lived asymmetric or "symmetry-broken" intermediate during the HA conformational change. This intermediate conformation is consistent with models of hemifusion, and its early formation during the conformational change has implications for the aggregation seen in HA activity.
Figures

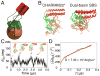

Similar articles
-
Exploring the early stages of the pH-induced conformational change of influenza hemagglutinin.Proteins. 2014 Oct;82(10):2412-28. doi: 10.1002/prot.24606. Epub 2014 Jun 9. Proteins. 2014. PMID: 24854389
-
Early steps of the conformational change of influenza virus hemagglutinin to a fusion active state: stability and energetics of the hemagglutinin.Biochim Biophys Acta. 2003 Jul 11;1614(1):3-13. doi: 10.1016/s0005-2736(03)00158-5. Biochim Biophys Acta. 2003. PMID: 12873761 Review.
-
Atomistic simulations indicate the functional loop-to-coiled-coil transition in influenza hemagglutinin is not downhill.Proc Natl Acad Sci U S A. 2018 Aug 21;115(34):E7905-E7913. doi: 10.1073/pnas.1805442115. Epub 2018 Jul 16. Proc Natl Acad Sci U S A. 2018. PMID: 30012616 Free PMC article.
-
Structural intermediates in the low pH-induced transition of influenza hemagglutinin.PLoS Pathog. 2020 Nov 30;16(11):e1009062. doi: 10.1371/journal.ppat.1009062. eCollection 2020 Nov. PLoS Pathog. 2020. PMID: 33253316 Free PMC article.
-
Receptor binding and membrane fusion in virus entry: the influenza hemagglutinin.Annu Rev Biochem. 2000;69:531-69. doi: 10.1146/annurev.biochem.69.1.531. Annu Rev Biochem. 2000. PMID: 10966468 Review.
Cited by
-
Dynamics of hemagglutinin-mediated membrane fusion.Proc Natl Acad Sci U S A. 2018 Aug 28;115(35):8655-8657. doi: 10.1073/pnas.1811183115. Epub 2018 Aug 20. Proc Natl Acad Sci U S A. 2018. PMID: 30127027 Free PMC article. No abstract available.
-
The pH-sensitive action of cholesterol-conjugated peptide inhibitors of influenza virus.Biochim Biophys Acta Biomembr. 2021 Dec 1;1863(12):183762. doi: 10.1016/j.bbamem.2021.183762. Epub 2021 Sep 1. Biochim Biophys Acta Biomembr. 2021. PMID: 34478733 Free PMC article.
-
Structural monitoring of a transient intermediate in the hemagglutinin fusion machinery on influenza virions.Sci Adv. 2020 Apr 29;6(18):eaaz8822. doi: 10.1126/sciadv.aaz8822. eCollection 2020 May. Sci Adv. 2020. PMID: 32494683 Free PMC article.
-
Insights into structural and inhibitory mechanisms of low pH-induced conformational change of influenza HA2 protein: a computational approach.J Mol Model. 2019 Mar 23;25(4):99. doi: 10.1007/s00894-019-3982-y. J Mol Model. 2019. PMID: 30904969
-
A conserved histidine in Group-1 influenza subtype hemagglutinin proteins is essential for membrane fusion activity.Virology. 2019 Oct;536:78-90. doi: 10.1016/j.virol.2019.08.005. Epub 2019 Aug 6. Virology. 2019. PMID: 31401467 Free PMC article.
References
-
- Skehel JJ, Wiley DC. Receptor Binding and Membrane Fusion in Virus Entry: The Influenza Hemagglutinin. Annual Review of bioc. 2000;69:531–569. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

