Prior human papillomavirus-16/18 AS04-adjuvanted vaccination prevents recurrent high grade cervical intraepithelial neoplasia after definitive surgical therapy: Post-hoc analysis from a randomized controlled trial
- PMID: 27541373
- PMCID: PMC5412942
- DOI: 10.1002/ijc.30391
Prior human papillomavirus-16/18 AS04-adjuvanted vaccination prevents recurrent high grade cervical intraepithelial neoplasia after definitive surgical therapy: Post-hoc analysis from a randomized controlled trial
Abstract
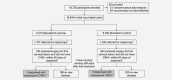
We evaluated the efficacy of the human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine in preventing HPV-related disease after surgery for cervical lesions in a post-hoc analysis of the PApilloma TRIal against Cancer In young Adults (PATRICIA; NCT00122681). Healthy women aged 15-25 years were randomized (1:1) to receive vaccine or control at months 0, 1 and 6 and followed for 4 years. Women were enrolled regardless of their baseline HPV DNA status, HPV-16/18 serostatus, or cytology, but excluded if they had previous or planned colposcopy. The primary and secondary endpoints of PATRICIA have been reported previously; the present post-hoc analysis evaluated efficacy in a subset of women who underwent an excisional procedure for cervical lesions after vaccination. The main outcome was the incidence of subsequent HPV-related cervical intraepithelial neoplasia grade 2 or greater (CIN2+) 60 days or more post-surgery. Other outcomes included the incidence of HPV-related CIN1+, and vulvar or vaginal intraepithelial neoplasia (VIN/VaIN) 60 days or more post-surgery. Of the total vaccinated cohort of 18,644 women (vaccine = 9,319; control = 9,325), 454 (vaccine = 190, control = 264) underwent an excisional procedure during the trial. Efficacy 60 days or more post-surgery for a first lesion, irrespective of HPV DNA results, was 88.2% (95% CI: 14.8, 99.7) against CIN2+ and 42.6% (-21.1, 74.1) against CIN1+. No VIN was reported and one woman in each group had VaIN2+ 60 days or more post-surgery. Women who undergo surgical therapy for cervical lesions after vaccination with the HPV-16/18 vaccine may continue to benefit from vaccination, with a reduced risk of developing subsequent CIN2+.
Keywords: cervical intraepithelial neoplasia; clinical trial; human papillomavirus vaccine; treatment.
© 2016 UICC.
Figures

References
-
- Walboomers JM, Jacobs MV, Manos MM, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999;189:12–9. - PubMed
-
- Li N, Franceschi S, Howell‐Jones R, et al. Human papillomavirus type distribution in 30,848 invasive cervical cancers worldwide: variation by geographical region, histological type and year of publication. Int J Cancer 2010;128:927–35. - PubMed
-
- de Sanjosé S, Quint WG, Alemany L, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross‐sectional worldwide study. Lancet Oncol 2010;11:1048–56. - PubMed
-
- Paavonen J, Jenkins D, Bosch FX, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus‐like‐particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double‐blind, randomised controlled trial. Lancet 2007;369:2161–70. - PubMed
-
- Paavonen J, Naud P, Salmeron J, et al. Efficacy of human papillomavirus (HPV)−16/18 AS04‐adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double‐blind, randomised study in young women. Lancet 2009;374:301–14. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

