The Association Between Patient-Reported and Objective Oral Anticancer Medication Adherence Measures: A Systematic Review
- PMID: 27541550
- PMCID: PMC5008846
- DOI: 10.1188/16.ONF.576-582
The Association Between Patient-Reported and Objective Oral Anticancer Medication Adherence Measures: A Systematic Review
Abstract
Problem identification: Oral anticancer medication (OAM) use has been steadily increasing, leading to several patient benefits. A notable challenge for nurses is accurate monitoring of patient OAM regimens because nonadherence is associated with poor health outcomes and decreased survival. Currently, no gold standard measure of OAM adherence exists. The authors conducted a systematic review of the association between objective and patient-reported measures of OAM adherence. .
Literature search: A systematic electronic literature search was conducted using PubMed, EMBASE, Scopus, PsycINFO®, Cochrane Library, Web of Science, and CINAHL® databases through November 2014. .
Data evaluation: Articles were independently reviewed to determine whether they included an original characterization of the level of association between objective and patient-reported measures of OAM adherence. .
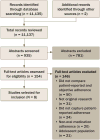
Synthesis: From a total of 11,135 articles retrieved, eight studies met inclusion criteria. Objective adherence was primarily assessed using pill counts or Medication Event Monitoring System (MEMSCap™). Patient-reported adherence was most commonly assessed using study-specific questionnaires. Significant positive correlations were observed between objective and patient-reported adherence across most studies, with three studies reporting higher rates of adherence via patient reporting. .
Conclusions: Despite variation in the OAMs and measures used, patient-reported adherence rates were equal to or higher than objective adherence measures across studies. Social desirability bias may be a concern; however, given the significant concordance observed, using patient-reported methods in future studies of OAM adherence may be justified. .
Implications for nursing: This review provides evidence to support nursing use of patient-reported measures to accurately monitor OAM adherence and potentially improve the quality of patient-provider communication.
Keywords: medication adherence; neoplasms; oral medicine; patient outcome assessment.
Figures
References
-
- Borner MM, Schoffski P, de Wit R, Caponigro F, Comella G, Sulkes A, et al. Fumoleau P. Patient preference and pharmacokinetics of oral modulated UFT versus intravenous fuorouracil and leucovorin: A randomised crossover trial in advanced colorectal cancer. European Journal of Cancer. 2002;38:349–358. doi: 10.1016/s0959-8049(01)00371-9. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous