Molecular Determinants of Tubulin's C-Terminal Tail Conformational Ensemble
- PMID: 27541566
- PMCID: PMC5745012
- DOI: 10.1021/acschembio.6b00507
Molecular Determinants of Tubulin's C-Terminal Tail Conformational Ensemble
Abstract
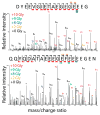
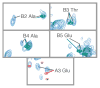
Tubulin is important for a wide variety of cellular processes including cell division, ciliogenesis, and intracellular trafficking. To perform these diverse functions, tubulin is regulated by post-translational modifications (PTM), primarily at the C-terminal tails of both the α- and β-tubulin heterodimer subunits. The tubulin C-terminal tails are disordered segments that are predicted to extend from the ordered tubulin body and may regulate both intrinsic properties of microtubules and the binding of microtubule associated proteins (MAP). It is not understood how either interactions with the ordered tubulin body or PTM affect tubulin's C-terminal tails. To probe these questions, we developed a method to isotopically label tubulin for C-terminal tail structural studies by NMR. The conformational changes of the tubulin tails as a result of both proximity to the ordered tubulin body and modification by mono- and polyglycine PTM were determined. The C-terminal tails of the tubulin dimer are fully disordered and, in contrast with prior simulation predictions, exhibit a propensity for β-sheet conformations. The C-terminal tails display significant chemical shift differences as compared to isolated peptides of the same sequence, indicating that the tubulin C-terminal tails interact with the ordered tubulin body. Although mono- and polyglycylation affect the chemical shift of adjacent residues, the conformation of the C-terminal tail appears insensitive to the length of polyglycine chains. Our studies provide important insights into how the essential disordered domains of tubulin function.
Conflict of interest statement
The authors declare no competing financial interest.
Figures






References
-
- Tompa P, Fuxreiter M. Fuzzy complexes: polymorphism and structural disorder in protein–protein interactions. Trends Biochem Sci. 2008;33:2–8. - PubMed
-
- Uversky VN. Multitude of binding modes attainable by intrinsically disordered proteins: a portrait gallery of disorder-based complexes. Chem Soc Rev. 2011;40:1623. - PubMed
-
- Sugase K, Dyson HJ, Wright PE. Mechanism of coupled folding and binding of an intrinsically disordered protein. Nature. 2007;447:1021–1025. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

