Aluminium chloride promotes tumorigenesis and metastasis in normal murine mammary gland epithelial cells
- PMID: 27541736
- PMCID: PMC5095782
- DOI: 10.1002/ijc.30393
Aluminium chloride promotes tumorigenesis and metastasis in normal murine mammary gland epithelial cells
Abstract
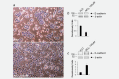
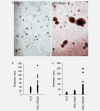
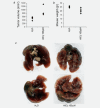
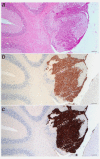
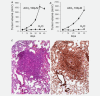
Aluminium salts, present in many industrial products of frequent use like antiperspirants, anti-acid drugs, food additives and vaccines, have been incriminated in contributing to the rise in breast cancer incidence in Western societies. However, current experimental evidence supporting this hypothesis is limited. For example, no experimental evidence that aluminium promotes tumorigenesis in cultured mammary epithelial cells exists. We report here that long-term exposure to concentrations of aluminium-in the form of aluminium chloride (AlCl3 )-in the range of those measured in the human breast, transform normal murine mammary gland (NMuMG) epithelial cells in vitro as revealed by the soft agar assay. Subcutaneous injections into three different mouse strains with decreasing immunodeficiency, namely, NOD SCID gamma (NSG), NOD SCID or nude mice, revealed that untreated NMuMG cells form tumors and metastasize, to a limited extent, in the highly immunodeficient and natural killer (NK) cell deficient NSG strain, but not in the less permissive and NK cell competent NOD SCID or nude strains. In contrast, NMuMG cells transformed in vitro by AlCl3 form large tumors and metastasize in all three mouse models. These effects correlate with a mutagenic activity of AlCl3 . Our findings demonstrate for the first time that concentrations of aluminium in the range of those measured in the human breast fully transform cultured mammary epithelial cells, thus enabling them to form tumors and metastasize in well-established mouse cancer models. Our observations provide experimental evidence that aluminium salts could be environmental breast carcinogens.
Keywords: aluminium; antiperspirants; breast cancer; carcinogen; metastasis; mouse models of cancer.
© 2016 The Authors International Journal of Cancer published by John Wiley & Sons Ltd on behalf of UICC.
Figures

Similar articles
-
Aluminium chloride promotes anchorage-independent growth in human mammary epithelial cells.J Appl Toxicol. 2012 Mar;32(3):233-43. doi: 10.1002/jat.1793. Epub 2012 Jan 6. J Appl Toxicol. 2012. PMID: 22223356
-
Effects of aluminium chloride and aluminium chlorohydrate on DNA repair in MCF10A immortalised non-transformed human breast epithelial cells.J Inorg Biochem. 2015 Nov;152:186-9. doi: 10.1016/j.jinorgbio.2015.08.003. Epub 2015 Aug 15. J Inorg Biochem. 2015. PMID: 26319584
-
Isolation and characterization of a spontaneously transformed malignant mouse mammary epithelial cell line in culture.Carcinogenesis. 1998 Nov;19(11):1907-11. doi: 10.1093/carcin/19.11.1907. Carcinogenesis. 1998. PMID: 9855001
-
Aluminium and the human breast.Morphologie. 2016 Jun;100(329):65-74. doi: 10.1016/j.morpho.2016.02.001. Epub 2016 Mar 17. Morphologie. 2016. PMID: 26997127 Review.
-
Aluminium and breast cancer: Sources of exposure, tissue measurements and mechanisms of toxicological actions on breast biology.J Inorg Biochem. 2013 Nov;128:257-61. doi: 10.1016/j.jinorgbio.2013.07.005. Epub 2013 Jul 13. J Inorg Biochem. 2013. PMID: 23899626 Review.
Cited by
-
Can Exposure to Environmental Pollutants Be Associated with Less Effective Chemotherapy in Cancer Patients?Int J Environ Res Public Health. 2022 Feb 12;19(4):2064. doi: 10.3390/ijerph19042064. Int J Environ Res Public Health. 2022. PMID: 35206262 Free PMC article. Review.
-
Use of Underarm Cosmetic Products in Relation to Risk of Breast Cancer: A Case-Control Study.EBioMedicine. 2017 Jul;21:79-85. doi: 10.1016/j.ebiom.2017.06.005. Epub 2017 Jun 6. EBioMedicine. 2017. PMID: 28629908 Free PMC article.
-
The postulated innocuity of lifetime exposure to aluminium should be reappraised.Front Oncol. 2023 Jul 24;13:1159899. doi: 10.3389/fonc.2023.1159899. eCollection 2023. Front Oncol. 2023. PMID: 37554161 Free PMC article.
-
Genomic Instability Is an Early Event in Aluminium-Induced Tumorigenesis.Int J Mol Sci. 2020 Dec 7;21(23):9332. doi: 10.3390/ijms21239332. Int J Mol Sci. 2020. PMID: 33297592 Free PMC article.
-
Aluminum Enters Mammalian Cells and Destabilizes Chromosome Structure and Number.Int J Mol Sci. 2021 Sep 1;22(17):9515. doi: 10.3390/ijms22179515. Int J Mol Sci. 2021. PMID: 34502420 Free PMC article.
References
-
- Exley C. Human exposure to aluminium. Environ Sci Process Impacts 2013; 15:1807–16. - PubMed
-
- Darbre PD. Aluminium, antiperspirants and breast cancer. J Inorg Biochem 2005; 99:1912–9. - PubMed
-
- Darbre PD. Metalloestrogens: an emerging class of inorganic xenoestrogens with potential to add to the oestrogenic burden of the human breast. J Appl Toxicol 2006; 26:191–7. - PubMed
-
- Banasik A, Lankoff A, Piskulak A, et al. Aluminum‐induced micronuclei and apoptosis in human peripheral‐blood lymphocytes treated during different phases of the cell cycle. Environ Toxicol 2005; 20:402–6. - PubMed
-
- Lankoff A, Banasik A, Duma A, et al. A comet assay study reveals that aluminium induces DNA damage and inhibits the repair of radiation‐induced lesions in human peripheral blood lymphocytes. Toxicol Lett 2006; 161:27–36. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

