MyD88 Shapes Vaccine Immunity by Extrinsically Regulating Survival of CD4+ T Cells during the Contraction Phase
- PMID: 27542117
- PMCID: PMC4991787
- DOI: 10.1371/journal.ppat.1005787
MyD88 Shapes Vaccine Immunity by Extrinsically Regulating Survival of CD4+ T Cells during the Contraction Phase
Abstract
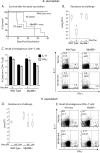
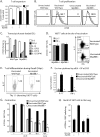
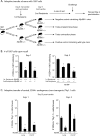
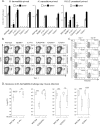
Soaring rates of systemic fungal infections worldwide underscore the need for vaccine prevention. An understanding of the elements that promote vaccine immunity is essential. We previously reported that Th17 cells are required for vaccine immunity to the systemic dimorphic fungi of North America, and that Card9 and MyD88 signaling are required for the development of protective Th17 cells. Herein, we investigated where, when and how MyD88 regulates T cell development. We uncovered a novel mechanism in which MyD88 extrinsically regulates the survival of activated T cells during the contraction phase and in the absence of inflammation, but is dispensable for the expansion and differentiation of the cells. The poor survival of activated T cells in Myd88-/- mice is linked to increased caspase3-mediated apoptosis, but not to Fas- or Bim-dependent apoptotic pathways, nor to reduced expression of the anti-apoptotic molecules Bcl-2 or Bcl-xL. Moreover, TLR3, 7, and/or 9, but not TLR2 or 4, also were required extrinsically for MyD88-dependent Th17 cell responses and vaccine immunity. Similar MyD88 requirements governed the survival of virus primed T cells. Our data identify unappreciated new requirements for eliciting adaptive immunity and have implications for designing vaccines.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Mochon AB, Cutler JE (2005) Is a vaccine needed against Candida albicans? Med Mycol 43: 97–115. - PubMed
-
- Deepe GS Jr., Wuthrich M, Klein BS (2005) Progress in vaccination for histoplasmosis and blastomycosis: coping with cellular immunity. Med Mycol 43: 381–389. - PubMed
-
- Cole GT, Xue JM, Okeke CN, Tarcha EJ, Basrur V, et al. (2004) A vaccine against coccidioidomycosis is justified and attainable. Med Mycol 42: 189–216. - PubMed
-
- Deepe GS Jr. (2004) Preventative and therapeutic vaccines for fungal infections: from concept to implementation. Expert Rev Vaccines 3: 701–709. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

