TGF-β1 stimulates migration of type II endometrial cancer cells by down-regulating PTEN via activation of SMAD and ERK1/2 signaling pathways
- PMID: 27542208
- PMCID: PMC5308649
- DOI: 10.18632/oncotarget.11311
TGF-β1 stimulates migration of type II endometrial cancer cells by down-regulating PTEN via activation of SMAD and ERK1/2 signaling pathways
Abstract
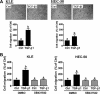
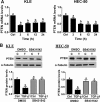
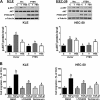
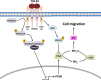
PTEN acts as a tumor suppressor primarily by antagonizing the PI3K/AKT signaling pathway. PTEN is frequently mutated in human cancers; however, in type II endometrial cancers its mutation rate is very low. Overexpression of TGF-β1 and its receptors has been reported to correlate with metastasis of human cancers and reduced survival rates. Although TGF-β1 has been shown to regulate PTEN expression through various mechanisms, it is not yet known if the same is true in type II endometrial cancer. In the present study, we show that treatment with TGF-β1 stimulates the migration of two type II endometrial cancer cell lines, KLE and HEC-50. In addition, TGF-β1 treatment down-regulates both mRNA and protein levels of PTEN. Overexpression of PTEN or inhibition of PI3K abolishes TGF-β1-stimulated cell migration. TGF-β1 induces SMAD2/3 phosphorylation and knockdown of common SMAD4 inhibits the suppressive effects of TGF-β1 on PTEN mRNA and protein. Interestingly, TGF-β1 induces ERK1/2 phosphorylation and pre-treatment with a MEK inhibitor attenuates the suppression of PTEN protein, but not mRNA, by TGF-β1. This study provides important insights into the molecular mechanisms mediating TGF-β1-induced down-regulation of PTEN and demonstrates an important role of PTEN in the regulation of type II endometrial cancer cell migration.
Keywords: PTEN; TGF-β1; migration; type II endometrial cancer.
Conflict of interest statement
None.
Figures



Similar articles
-
Activin B promotes endometrial cancer cell migration by down-regulating E-cadherin via SMAD-independent MEK-ERK1/2-SNAIL signaling.Oncotarget. 2016 Jun 28;7(26):40060-40072. doi: 10.18632/oncotarget.9483. Oncotarget. 2016. PMID: 27223076 Free PMC article.
-
Differential roles of Smad2 and Smad3 in the regulation of TGF-β1-mediated growth inhibition and cell migration in pancreatic ductal adenocarcinoma cells: control by Rac1.Mol Cancer. 2011 May 30;10:67. doi: 10.1186/1476-4598-10-67. Mol Cancer. 2011. PMID: 21624123 Free PMC article.
-
Activation of extracellular signal-regulated kinase by TGF-beta1 via TbetaRII and Smad7 dependent mechanisms in human bronchial epithelial BEP2D cells.Cell Biol Toxicol. 2007 Mar;23(2):113-28. doi: 10.1007/s10565-006-0097-x. Epub 2006 Nov 9. Cell Biol Toxicol. 2007. PMID: 17096210
-
Role of Tumor Suppressor PTEN and Its Regulation in Malignant Transformation of Endometrium.Biochemistry (Mosc). 2022 Nov;87(11):1310-1326. doi: 10.1134/S0006297922110104. Biochemistry (Mosc). 2022. PMID: 36509719 Review.
-
PTEN regulation of ERK1/2 signaling in cancer.J Recept Signal Transduct Res. 2012 Aug;32(4):190-5. doi: 10.3109/10799893.2012.695798. Epub 2012 Jun 28. J Recept Signal Transduct Res. 2012. PMID: 22737980 Free PMC article. Review.
Cited by
-
NLRP3 in human glioma is correlated with increased WHO grade, and regulates cellular proliferation, apoptosis and metastasis via epithelial-mesenchymal transition and the PTEN/AKT signaling pathway.Int J Oncol. 2018 Sep;53(3):973-986. doi: 10.3892/ijo.2018.4480. Epub 2018 Jul 12. Int J Oncol. 2018. PMID: 30015880 Free PMC article.
-
The m6A reader YTHDC1-mediated lncRNA CTBP1-AS2 m6A modification accelerates cholangiocarcinoma progression.Heliyon. 2023 Sep 4;9(9):e19816. doi: 10.1016/j.heliyon.2023.e19816. eCollection 2023 Sep. Heliyon. 2023. PMID: 37809459 Free PMC article.
-
MicroRNA‑125a‑5p controls the proliferation, apoptosis, migration and PTEN/MEK1/2/ERK1/2 signaling pathway in MCF‑7 breast cancer cells.Mol Med Rep. 2019 Nov;20(5):4507-4514. doi: 10.3892/mmr.2019.10704. Epub 2019 Sep 24. Mol Med Rep. 2019. PMID: 31702027 Free PMC article.
-
Estrogen inhibits autophagy and promotes growth of endometrial cancer by promoting glutamine metabolism.Cell Commun Signal. 2019 Aug 20;17(1):99. doi: 10.1186/s12964-019-0412-9. Cell Commun Signal. 2019. PMID: 31429768 Free PMC article.
-
miR372 Promotes Progression of Liver Cancer Cells by Upregulating erbB-2 through Enhancement of YB-1.Mol Ther Nucleic Acids. 2018 Jun 1;11:494-507. doi: 10.1016/j.omtn.2018.04.001. Epub 2018 Apr 12. Mol Ther Nucleic Acids. 2018. PMID: 29858084 Free PMC article.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. - PubMed
-
- Bokhman JV. Two pathogenetic types of endometrial carcinoma. Gynecol Oncol. 1983;15:10–17. - PubMed
-
- Murali R, Soslow RA, Weigelt B. Classification of endometrial carcinoma: more than two types. Lancet Oncol. 2014;15:e268–278. - PubMed
-
- Di Cristofano A, Ellenson LH. Endometrial carcinoma. Annu Rev Pathol. 2007;2:57–85. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

